home > Pablication
2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
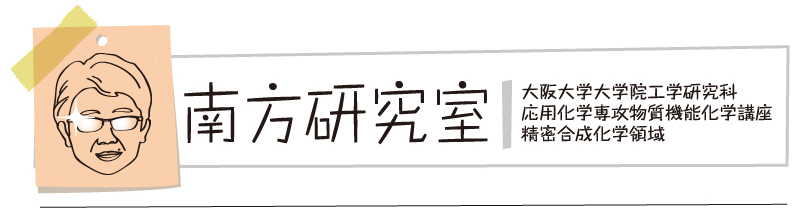
“Dibenzophenazine-Based TADF Emitters as Dual Electrochromic and Electroluminescence Materials"
Paola Zimmermann Crocomo, Masato Okazaki, Takumi Hosono, Satoshi Minakata, Youhei Takeda*, and Przemyslaw Data*
Chem. Eur. J. 2022, 28, e202200826/1–8. DOI:10.1002/chem.202200826
Abstract: The change of donors in CT-based molecules significantly influences the charge carrier generation and follow-up reactions that affect the TADF emitter‘s stability. The TADF emitters can be easily electropolymerised, forming stable conjugated polymers. Electrochemically active TADF emitters possess significant electrochromic properties and can be used as both electrochromic and electroluminescence materials. The dual behaviour of the TADF emitters allows use in both OLED and Electrochromic Windows applications.
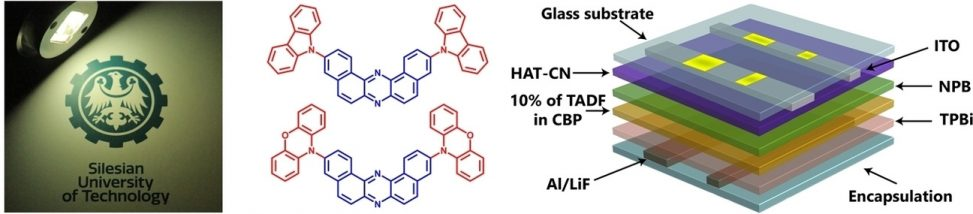
“Comparative Study of Thermally Activated Delayed Fluorescent Properties of Donor–Acceptor and Donor–Acceptor–Donor Architectures Based on Phenoxazine and Dibenzo[a,j]phenazine"
Saika Izumi, Prasannamani Govindharaj, Anna Drewniak, Paola Zimmermann Crocomo, Satoshi Minakata1, Leonardo Evaristo de Sousa, Piotr de Silva*, Przemyslaw Data*, and Youhei Takeda*
Beilstein J. Org. Chem. 2022, 18, 459–468. DOI:10.3762/bjoc.18.48
*Open-Access Article!
*Invited as a part of the themed collection "Organic TADF materials design"!
Abstract: A new thermally activated delayed fluorescence (TADF) compound based on a donor–acceptor (D–A) architecture (D = phenoxazine; A = dibenzo[a,j]phenazine) has been developed, and its photophysical properties were characterized. The D–A compound is applicable as an emitting material for efficient organic light-emitting diodes (OLEDs), and its external quantum efficiency (EQE) exceeds the theoretical maximum of those with prompt fluorescent emitters. Most importantly, comparative study of the D–A molecule and its D–A–D counterpart from the viewpoints of the experiments and theoretical calculations revealed the effect of the number of the electron donor on the thermally activated delayed fluorescent behavior.
“Dual-Photofunctional Organogermanium Compound Based on Donor–Acceptor–Donor Architecturse"
Aleksandra Nyga, Takahito Kaihara, Takumi Hosono, Massimiliano Sipala, Patrycja Stachelek, Norimitsu Tohnai, Satoshi Minakata, Leonardo Evaristo de Sousa, Piotr de Silva*, Przemyslaw Data*, and Youhei Takeda*
Chem. Commun. 2022, 58, 5889–5892. DOI:10.1039/D2CC01568D
*Open-Access Article!
*Invited as a part of the themed collection "2022 Pioneering Investigators"!
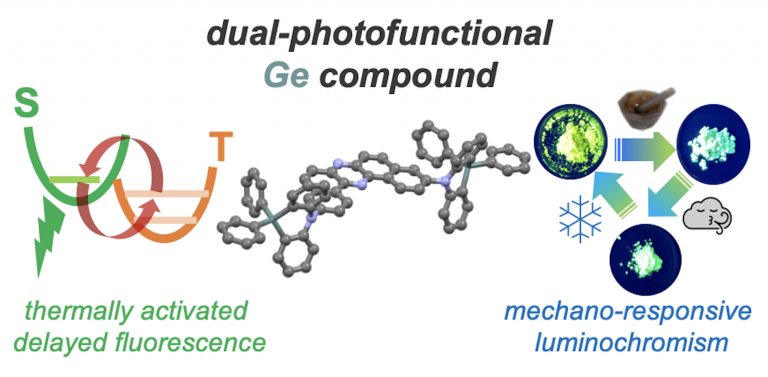
Abstract: A dual-photofunctional organogermanium compound based on a donor–acceptor–donor architecture that exhibits thermally activated delayed fluorescence and mechano-responsive luminochromism has been developed. The developed compound was successfully applied as an emitter for efficient organic light-emitting diodes.
“Regioisomeric Effect on the Excited-State Fate Leading to Room-Temperature Phosphorescence or Thermally Activated Delayed Fluorescence in a Dibenzophenazine-Cored Donor–Acceptor–Donor System"
Takumi Hosono, Nicolas Oliveira Decarli, Paola Zimmermann Crocomo, Tsuyoshi Goya, Leonardo Evaristo de Sousa, Norimitsu Tohnai, Satoshi Minakata, Piotr de Silva*, Przemyslaw Data*, and Youhei Takeda*
J. Mater. Chem. C 2022 10, 4905–4913. DOI:10.1039/D1TC05730H
*Invited as a part of the themed collection "Materials for thermally activated delayed fluorescence and/or triplet fusion upconversion"!
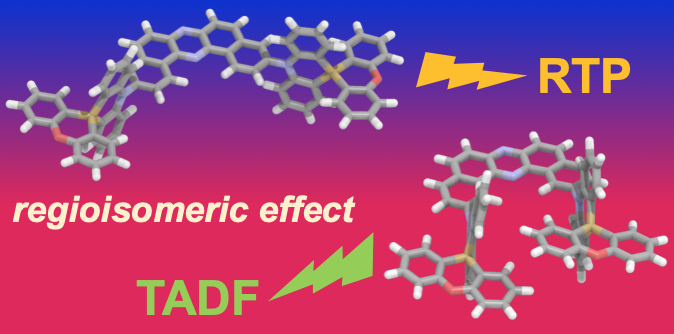
Abstract: Exploring the design principle for switching between thermally activated delayed fluorescence (TADF) and room temperature phosphorescence (RTP) is a fundamentally important research to develop triplet-mediated photofunctional organic materials. Herein systematic studies on the regioisomeric and substituent effects in a twisted donor–acceptor–donor (D–A–D) molecular scaffold (A = dibenzo[a,j]phenazine; D = dihydrophenazasiline) on the fate of its excited state have been performed. The study revealed that regioisomerism clearly affects the emission behavior of the D–A–D compounds. Moreover, distinct differences in TADF, dual TADF & RTP, and dual RTP were observed, depending on the host used. Furthermore, organic light-emitting diodes (OLEDs) fabricated with the developed emitters achieved high external quantum efficiencies (EQEs) of up to 7.4% for RTP-based OLEDs.
“A New Entry to Purely Organic Thermally Activated Delayed Fluorescence Emitters Based on Pyrido[2,3-b]pyrazine–Dihydrophenazasilines Donor–Acceptor Dyad"
Tsuyoshi Goya, Paola Zimmermann Crocomo, Takumi Hosono, Satoshi Minakata, Leonardo Evaristo de Sousa, Piotr de Silva*, Przemyslaw Data*, and Youhei Takeda*
Asian J. Org. Chem. 2022 11, e202100890/1–8. DOI:10.1002/ajoc.202100780 (Published as part of the 10th Anniversary Collection-invitaion only!)
*Open-Access Article!
*Invited as a part of the Special Collection "10th Anniversay Collection"!
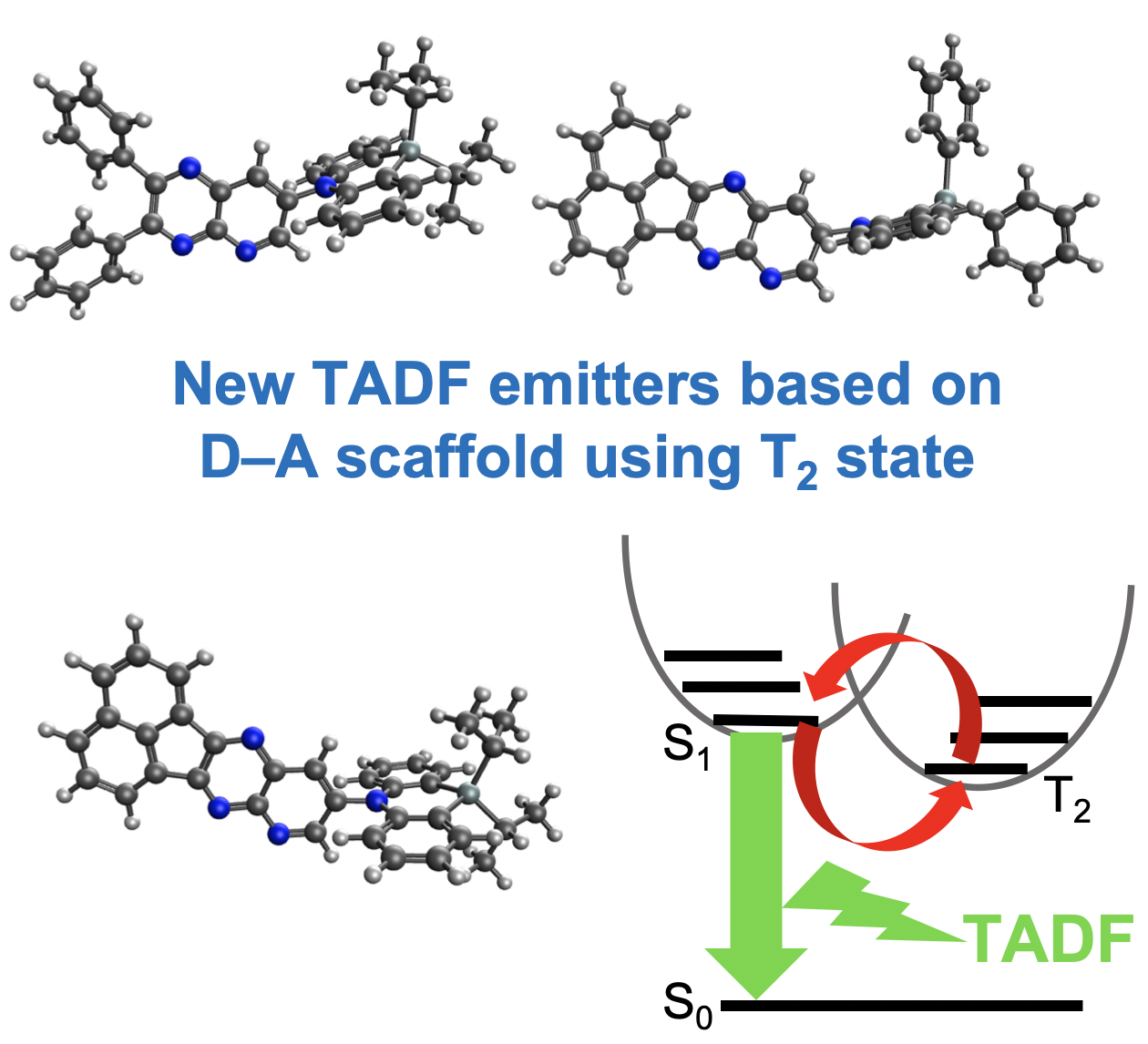
Abstract: A new family of thermally activated delayed fluorescence (TADF) emitters based on a twisted donor–acceptor (D–A) dyad scaffold comprising of dihydrophenazasiline (D) and pyrido[2,3- b ]pyrazine (A) has been developed, and their properties have been investigated. Time-resolved spectroscopic analysis in matrices revealed the detailed photophysical properties of the D–A compounds. These D–A compounds serve as the emitter for organic light-emitting diodes (OLEDs), showing a moderate external quantum efficiency (EQE) up to 9% in CBP matrix. Furthermore, theoretical calculations uncovered the excited states nature of the developed TADF emitters.