2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
"Oxidative Dimerization of (Hetero)aromatic Amines Utilizing t‐BuOI Leading to (Hetero)aromatic Azo Compounds: Scope and Mechanistic Studies"
Sota Okumura, Chun-Hsuan Lin, Youhei Takeda*, and Satoshi Minakata*
J. Org. Chem.. 2013, 78, 12090–12105. DOI: 10.1021/jo402120w
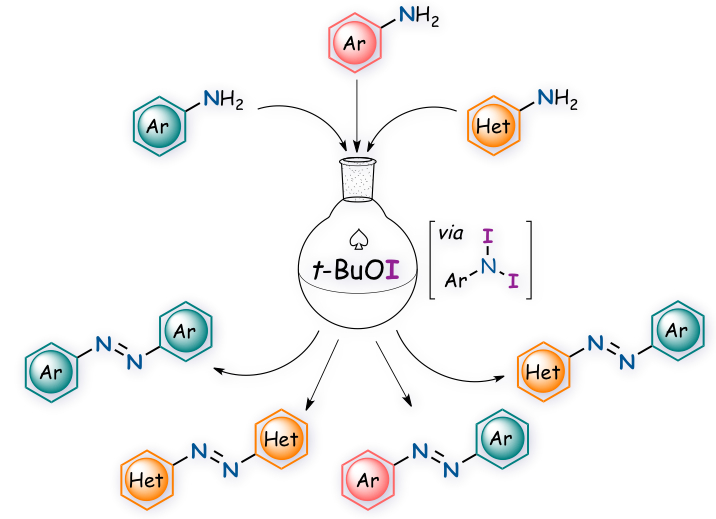
Abstract: A straightforward synthetic method of both symmetric and unsymmetric aromatic azo compounds through an efficient and cross-selective oxidative dimerization of aromatic amines using tert-butyl hypoiodite (t-BuOI) under metal-free and mild conditions has been developed. This method was also found applicable to the synthesis of heteroaromatic azo compounds. The spectroscopic study indicates the involvement of N,N-diiodoanilines in the oxidative reaction as the key intermediate.
"Metal-Free Aziridination of Styrene Derivatives with Iminoiodinane Catalyzed by a Combination of Iodine and Ammonium Iodide"
Kensuke Kiyokawa, Tomoki Kosaka, and Satoshi Minakata*
Org. Lett. 2013, 15, 4858–4861. DOI: 10.1021/ol402276f
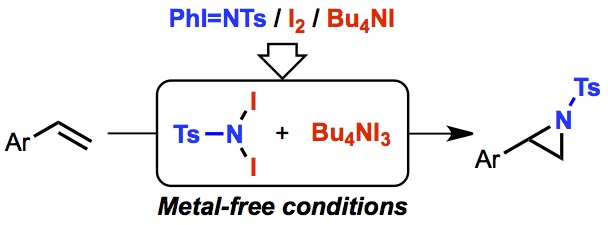
Abstract: The metal-free catalytic aziridination of styrene derivatives with N-tosyliminophenyliodinane (PhI=NTs) in the presence of a combination of I2 and tetrabutylammonium iodide (TBAI) is reported. In situ generated TBAI3 from I2 and TBAI dramatically promotes the reaction of alkenes with N,N-diiodotosylamide, which is formed in situ..
"PCy3-Catalyzed Ring-Expansion of Aziridinofullerenes with CO2 and Aryl Isocyanates: Evidence for a Two-Conseutive Nucleophilic Substitution Pathway on the Fullerene Cage"
Youhei Takeda, Hajime Kawai, and Satoshi Minakata*
Chem. Eur. J. 2013, 19, 13479–13483. DOI: 10.1002/chem.201301617
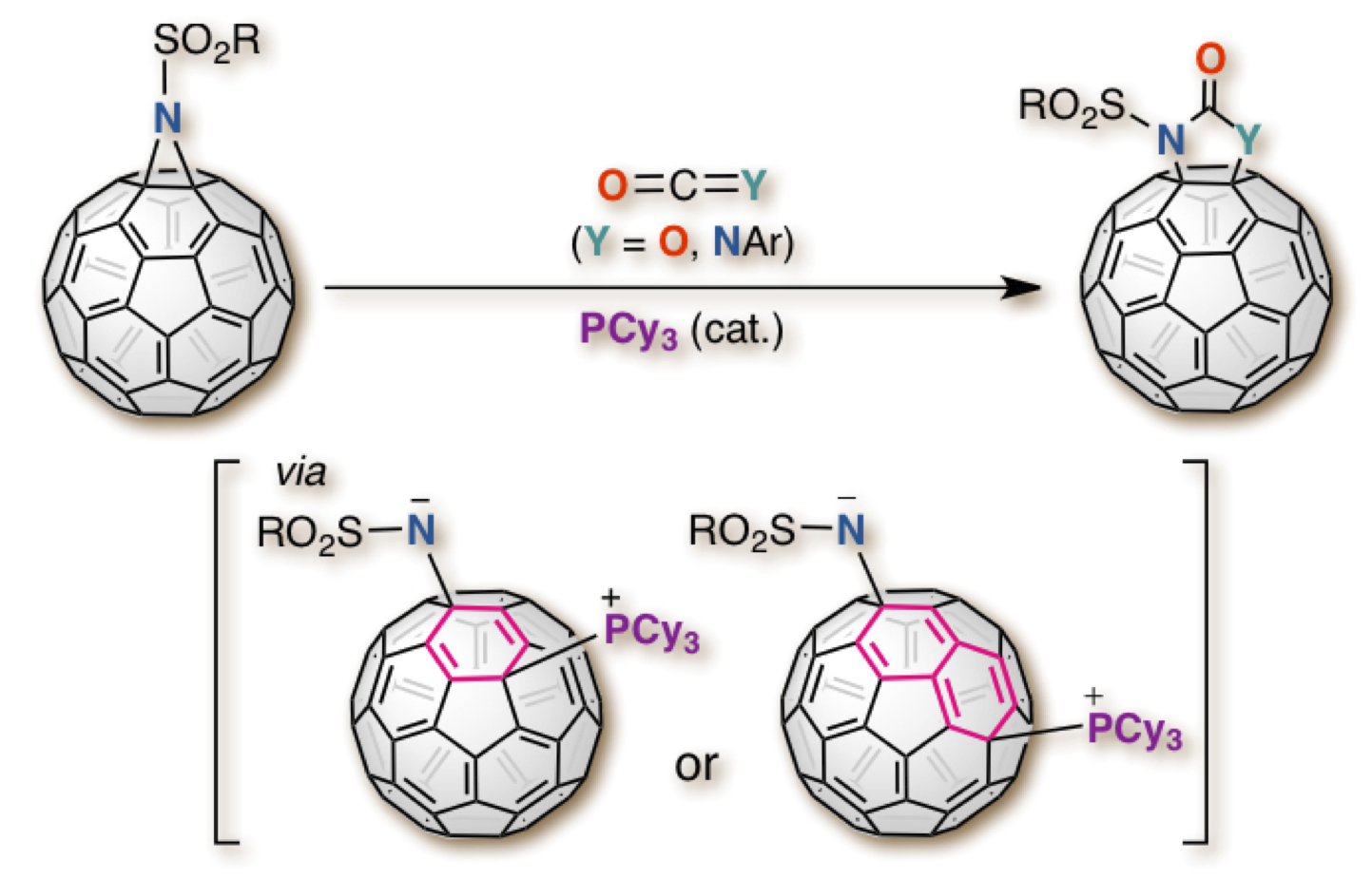
Abstract: A PCy3-catalyzed ring-expansion reaction of aziridine-fused fullerenes (aziridinofullerenes) through the insertion of CO2 and aryl isocyanates is disclosed. The reaction allows for CO2 fixation by aziridinofullerenes, producing oxazolidinone-fused fullerenes (oxazolidinofullerenes) in high yields, while the reaction with aryl isocyanates led to a new fullerene family, imidazolidinone-fused fullerenes (imidazolidinofullerenes), in good to high yields. Furthermore, a mechanistically related unprecedented fullerenyl phosphonium salt was successfully isolated. Using the isolated salt, mechanistic studies were also investigated.
"Hypervalent Iodine(III)-Induced Oxidative [4+2] Annulation of o-Phenylenediamines and Electron-Deficient Alkynes: Direct Synthesis of Quinoxalines from Alkyne Substrates under Metal-Free Conditions"
Sota Okumura, Youhei Takeda,* Kensuke Kiyokawa, and Satoshi Minakata*
Chem. Commun. 2013, 49, 9266–9268. DOI: 10.1039/C3CC45469J
* Open-access Article!
* Highlighted in Synfacts (2014, 10, 0038)!(see the detail)
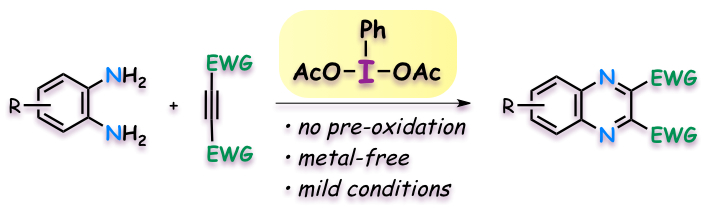
Abstract: Hypervalent iodine(III)-induced oxidative [4+2] annulation of o-phenylenediamines and electron-deficient alkynes under metal-free conditions has been developed. The reaction allows for direct access to quinoxalines bearing two electron-withdrawing groups in an efficient manner.
"A Practical Synthesis of Azobenzenes through Oxidative Dimerization of Aromatic Amines Using tert-Butyl Hypoiodite"
Youhei Takeda, Sota Okumura, and Satoshi Minakata*
Synthesis 2013, 45, 1029–1033. DOI: 10.1055/s-0032-1318388
* Invited as a "Practical Synthetic Procedures (PSP) articles"!
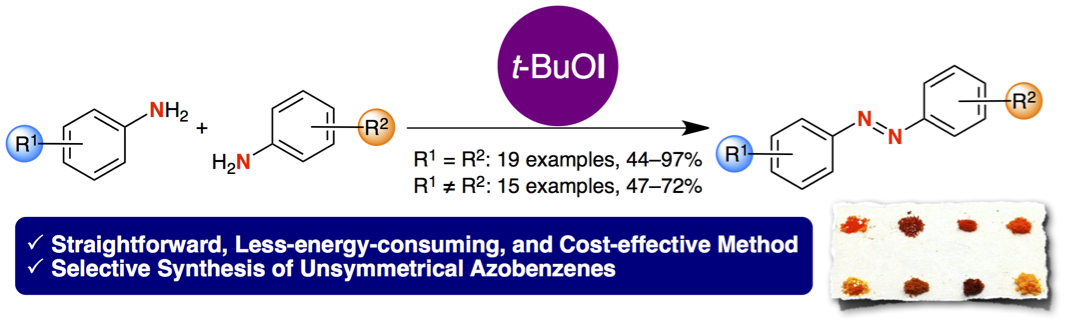
Abstract: A straightforward, convenient, and efficient synthetic method of azobenzenes through oxidative dimerization of aromatic amines using a unique and cost-effective iodinating reagent is described. This new method allows for easy access to both of symmetrical and unsymmetrical azobenzenes under extremely mild conditions.
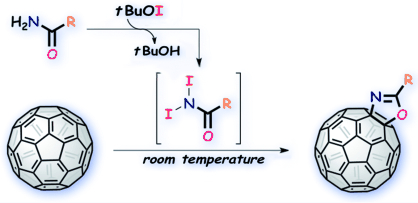
"Straightforward and Versatile Synthesis of Fullerooxazoles from C60 and Carboxamides through Radical Reactions under Mild Conditions"
Youhei Takeda, Satoru Enokijima, Toshiki Nagamachi, Kazuhisa Nakayama, and Satoshi Minakata*
Asian J. Org. Chem. 2013, 2, 91–97. DOI: 10.1021/ajoc.201200114
* Highlighted in ChemistryViews as a "Noteworthy Paper"! (see the detail)
* Highlighted in "ワイリー・サイエンスカフェ"!(link)
* Ranked as the most-accessed article for February, March, April and May 2013 in a row!(see the detail)
Abstract: A direct synthetic method for producing oxazoline-fused fullerenes, that is, fullerooxazoles, from [60] fullerene and readily available carboxamides by radical pathways has been developed. The method presented allows efficient access to a variety of fullerooxazoles with high functional compatibility under mild reaction conditions. Furthermore, systematic investigation of their properties, such as solubility, thermostability, and electrochemical behavior, was conducted.
"An Inclusion Complex of C60 with Organosilylated γ-Cyclodextrin: Drastic Enhancement of Apparent Solubility of C60 in Nonpolar and Weakly Polar Organic Solvents"
Youhei Takeda, Toshiki Nagamachi, Katsuaki Nishikori, and Satoshi Minakata*
Asian J. Org. Chem. 2013, 2, 69–73. DOI: 10.1021/ajoc.201200160
* Highlighted in ChemistryViews as a "Noteworthy Paper"! (see the detail)
* Highlighted in "ワイリー・サイエンスカフェ"!(link)
* Highlighted in “Angewandte Spotlights” (Angew. Chem. Int. Ed. 2013, 52, 796–798.)! (link)
* Ranked as one of the most-accessed articles (6th) for February 2013!(see the detail)
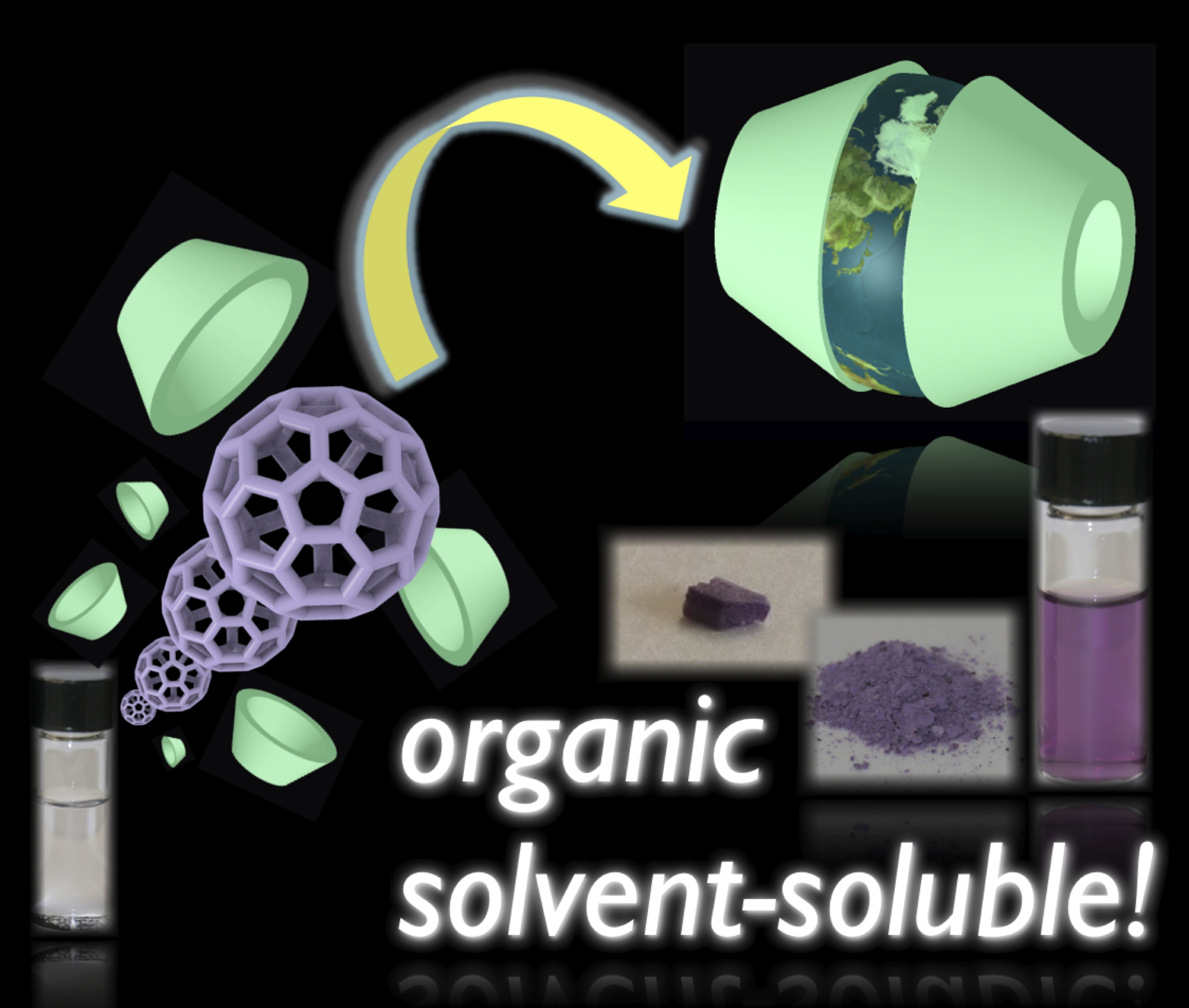
Abstract: A bi-capped inclusion complex of C60 with organosilylated γ-cyclodextrin that is highly soluble in nonpolar and weakly polar organic solvents, such as CHCl3 or cyclohexane, was prepared and characterized. The complex facilitates the separation of C60 from C70 and selective functionalization of C60. Furthermore, fabrication of a thin film of the complex by using a solution technique was demonstrated.