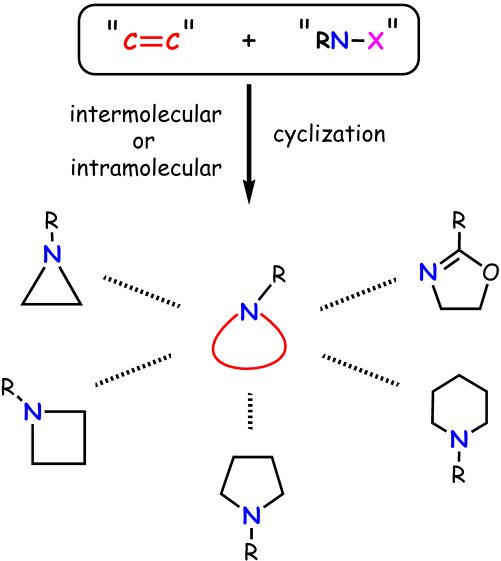
"Aziridination of C60 with Simple Amides and Catalytic Rearrangement of the Aziridinofullerenes to Azafulleroids"
Ryoji Tsuruoka, Toshiki Nagamachi, Yuta Murakami, Mitsuo Komatsu, and Satoshi Minakata*
J. Org. Chem. 2009,
74, 1691-1697. DOI:
10.1021/jo8025737
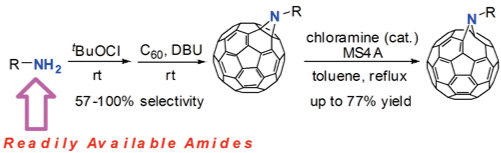
Abstract: The selective formation of aziridinofullerene and azafulleroid, which are isomers of the fullerene derivatives-introduced N
1 unit, is achieved. The ionic aziridination is a very convenient and risk-free procedure compared with the conventional method with azides as nitrogen sources, and gives aziridinofullerenes from various readily available amides (carbamates, ureas, carboxamides, and phosphamides). For example, benzyl carbamate was chlorinated by
tert-butyl hypochlorite (
tert-BuOCl) and then reacted with C
60 in the presence of base to give
N-benzyloxycarbonyl aziridinofullerene exclusively and without formation of its isomer, an azafulleroid. The reaction enabled the synthesis of functional fullerene derivatives having a trialkoxysilyl group and an amino acid moiety. Azafulleroids were obtained through the rearrangement of corresponding aziridinofullerenes by using the combination of a chloramine catalyst and MS4A. Among other chloramines used, chloramine B (CB) showed superior ability as a catalyst in the rearrangement. It was found that MS4A functions as a Lewis acid in the reaction.
"Asymmetric Recognition and Sequential Ring Opening of 2-Substituted-N-Nosylaziridines with (DHQD)2AQN and TMSNu"
Satoshi Minakata,* Yuta Murakami, Masamitsu Satake, Ikumasa Hidaka, Yuriko Okada
and Mitsuo Komatsu
Org. Biomol. Chem. 2009,
7, 641-643. DOI:
10.1039/b821650a
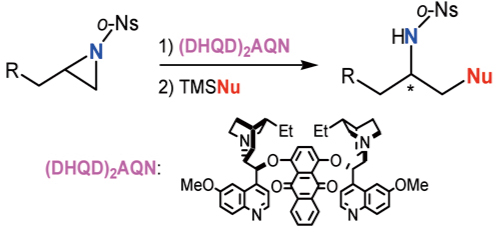
Abstract: A new method for asymmetric ring opening of terminal aziridines using a chiral amine, (DHQD)
2AQN, is described; the reaction is based on the asymmetric recognition of aziridines using (DHQD)
2AQN and on sequential ring opening using TMSNu.