2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
“Revealing Topological Influence of Phenylenediamine Unit on Physicochemical Properties of Donor‐Acceptor‐Donor‐Acceptor Thermally Activated Delayed Fluorescent Macrocycles"
Saika Izumi, Aleksandra Nyga, Piotr de Silva*, Norimitsu Tohnai, Satoshi Minakata*, Przemyslaw Data*, and Youhei Takeda*
Chem. Asian J. 2020, 15, 4098–4103. DOI:10.1002/asia.202001173
*Selected as Very Important Paper (VIP)!
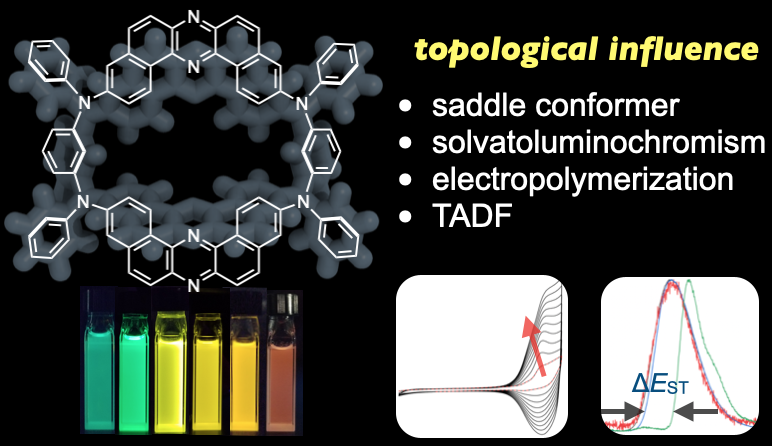
Abstract: A new thermally activated delayed fluorescence (TADF)‐displaying macrocyclic compound m‐1 comprising of two electron‐donors (N,N’‐diphenyl‐m‐phenylenediamine) and two electron‐acceptors (dibenzo[a,j]phenazine) has been synthesized. The macrocycle developed herein is regarded as a regioisomer of the previously reported TADF macrocycle p‐1, which has two N,N’‐diphenyl‐p‐phenylenediamines as the donors. To understand the influence of the topology of the phenylenediamine donors on physicochemical properties of TADF‐active macrocycles, herein the molecular structure in the single crystals, photophysical properties, electrochemical behavior, and TADF properties of m‐1 have been investigated compared with those of p‐1. The substitution of p‐phenylene donor with m‐phenylene donor led to distinct positive solvatoluminochromism over the full visible‐color range, unique oxidative electropolymerization, and slightly lower contribution of TADF, due to the lower CT character in the excited states.
“Electrochemical and Spectroelectrochemical Comparative Study of Macrocyclic Thermally Activated Delayed Fluorescent Compounds: Molecular Charge Stability vs OLED EQE Roll‐Off"
Aleksandra Nyga, Saika Izumi, Heather F. Higginbotham, Patrycja Stachelek, Sandra Pluczyk, Piotr de Silva*, Satoshi Minakata*, Youhei Takeda*, and Przemyslaw Data*
Asian J. Org. Chem. 2020, 9, 2153–2161. (Published as part of the Special Collection dedicated to early career researchers.) DOI:10.1002/ajoc.202000475
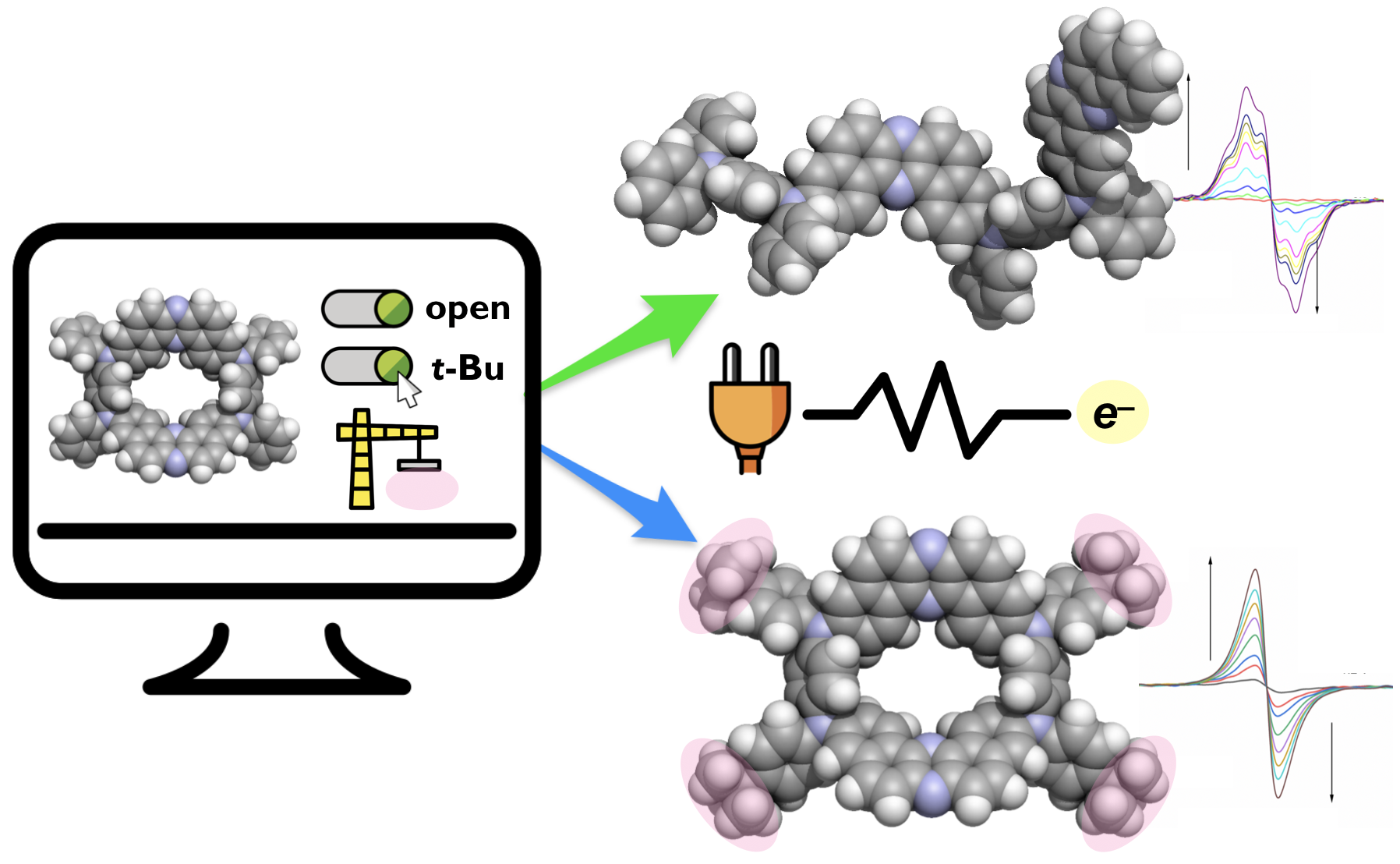
Abstract:In this work, we present how a small change in molecular structure can affect the electrochemical stability of organic compounds. A new electron donor‐acceptor‐donor‐acceptor (D‐A‐D‐A) macrocyclic π‐conjugated compound (tBuMC) comprising of dibenzophenazine as As and N,N’‐bis(t‐butylphenyl)‐p‐phenylenediamines as Ds has been synthesized. The photophysical investigation uncovered that tBuMC showed thermally activated delayed fluorescence and that the organic light‐emitting diodes (OLEDs) fabricated with tBuMC as the emitter achieved high external quantum efficiency (EQEs) of ca. 10%. However, the OLED with tBuMC showed a slightly lower EQE than that of the OLED with MC (11.6%) and showed greater EQE roll‐off. Comparative studies on electrochemical properties of tBuMC, MC, and a linear analogue (Linear) revealed the introduction of t‐Bu groups in the D‐A‐D‐A scaffold causes a significant change in redox behavior. Full electrochemical and spectroelectrochemical studies gave clues to understand how the steric hindering group is affecting the charge distribution in the new molecules which results in a significant difference in the OLED roll‐off. The electrochemical investigations together with UV‐Vis‐NIR and EPR analyses supported by quantum chemical theoretical calculations were performed, which provided us insights on the effect of structural modification on the redox properties of the D‐A‐D‐A scaffold.
“Transition-Metal-Free Aziridination of Alkenes with Sulfamate Esters Using tert-Butyl Hypoiodite"
Kensuke Kiyokawa*, Shogo Nakamura, and Satoshi Minakata*
Heterocycles 2021, 103, 190–197. (Published as part of the Special Issue in honor of Professor Yasuyuki Kita on his 77th birthday.) DOI:10.3987/COM-20-S(K)20
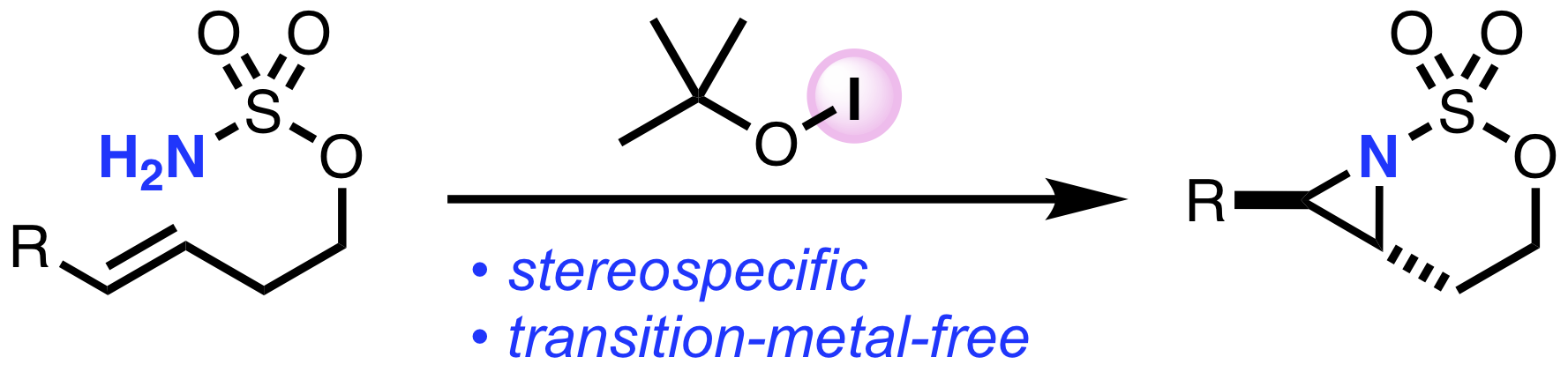
Abstract: The transition-metal-free aziridination of alkenes with sulfamate esters in the presence of tert-butyl hypoiodite (t-BuOI) is reported. The reaction can be used in intra- and intermolecular reactions, offering a practical and environmentally benign method for the synthesis of valuable aziridine compounds.
“Sigmoidally Hydrochromic Molecular Porous Crystal with Rotatable Dendrons"
Hiroshi Yamagishi, Sae Nakajima, Jooyoung Yoo, Masato Okazaki, Youhei Takeda*, Satoshi Minakata, Ken Albrecht*, Kimihisa Yamamoto, Irene Badía-Domínguez, Maria Moreno Oliva, M. Carmen Ruiz Delgado, Yuka Ikemoto, Hiroyasu Sato, Kenta Imoto, Kosuke Nakagawa, Hiroko Tokoro, Shin-ichi Ohkoshi, and Yohei Yamamoto*
Commun. Chem. 2020, 3, 118/1–8. DOI:10.1038/s42004-020-00364-3
*Open-access Article!
*Highlighted in Resou, Optronics, EurekAlert!, ScienceDaily, PhysOrg!
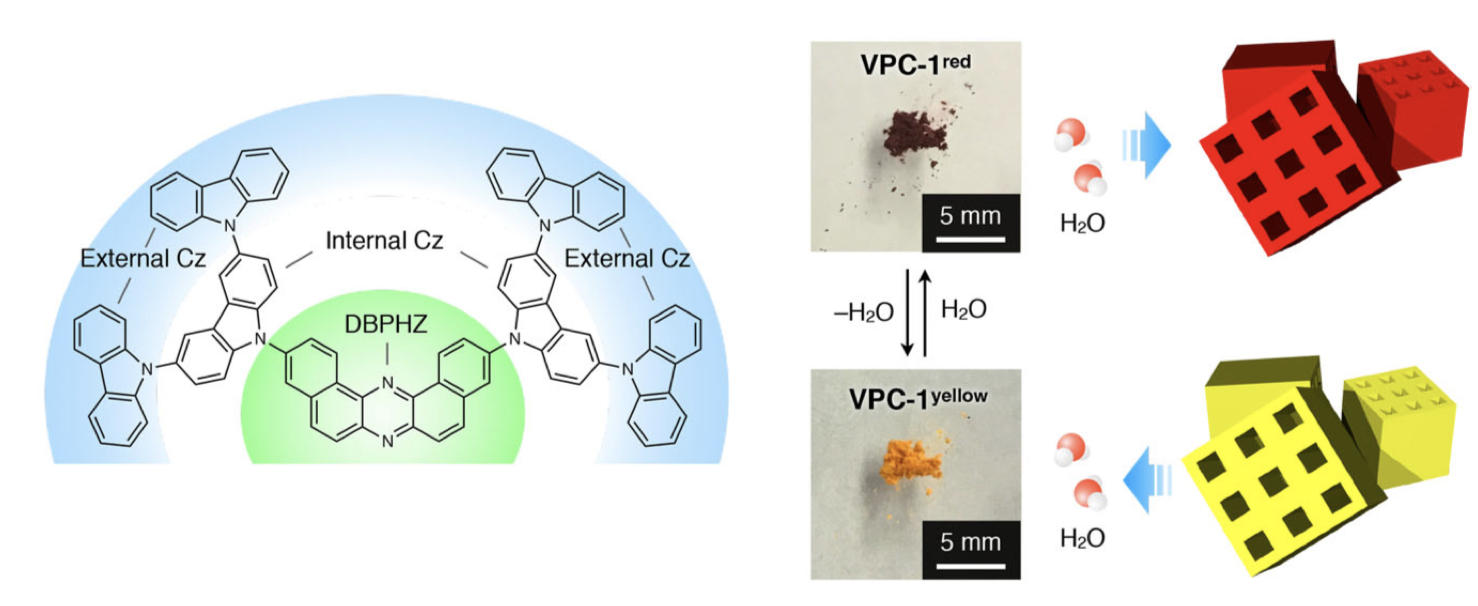
Abstract: Vapochromic behaviour of porous crystals is beneficial for facile and rapid detection of gaseous molecules without electricity. Toward this end, tailored molecular designs have been established for metal–organic, covalent-bonded and hydrogen-bonded frameworks. Here, we explore the hydrochromic chemistry of a van der Waals (VDW) porous crystal. The VDW porous crystal VPC-1 is formed from a novel aromatic dendrimer having a dibenzophenazine core and multibranched carbazole dendrons. Although the constituent molecules are connected via VDW forces, VPC-1 maintains its structural integrity even after desolvation. VPC-1 exhibits reversible colour changes upon uptake/release of water molecules due to the charge transfer character of the constituent dendrimer. Detailed structural analyses reveal that the outermost carbazole units alone are mobile in the crystal and twist simultaneously in response to water vapour. Thermodynamic analysis suggests that the sigmoidal water sorption is induced by the affinity alternation of the pore surface from hydrophobic to hydrophilic.
“Palladium-Catalyzed Regioselective and Stereospecific Ring-Opening Cross-Coupling of Aziridines: Experimental and Computational Studies"
Youhei Takeda*, W. M. C. Sameera*, and Satoshi Minakata*
Acc. Chem. Res. 2020, 53, 1686–1702. DOI:10.1021/acs.accounts.0c00395
*Open-access Article!
*Account Article!
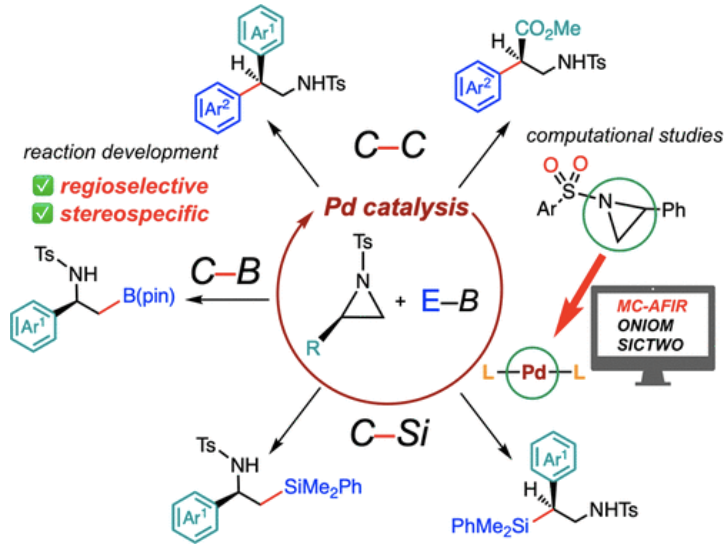
Abstract: In this Account, starting from the background of transition-metal-catalyzed ring-opening functionalization of aziridines, our contributions to the palladium-catalyzed regioselective and stereoinvertive cross-couplings of aziridines with organoboron reagents to form C(sp3)–C, C(sp3)–B, and C(sp3)–Si bonds have been compiled. The developed methods allow the syntheses of medicinally important amine compounds, e.g., enantioenriched β-phenethylamines, β-amino acids, and their boron and silyl surrogates, from readily available enantiopure aziridine substrates. Notably, the regioselectivity of the ring opening can be switched by appropriate selection of the catalyst (i.e., Pd/NHC vs Pd/PR3 systems). Computational studies rationalized the detailed mechanisms of the full catalytic cycle and the regioselectivity and stereospecificity of the reactions. The computational results suggested that the interactions operating between the Pd catalyst and aziridine substrate play important roles in determining the regioselection of the aziridine ring-opening event (i.e., oxidative addition). Also, the computational results rationalized the role of water molecules in promoting the transmetalation step through the formation of a Pd–hydroxide active intermediate. This Account evidences the benefits of synergistic collaborations between experimental and computational methods in developing novel transition-metal-catalyzed cross-coupling reactions.
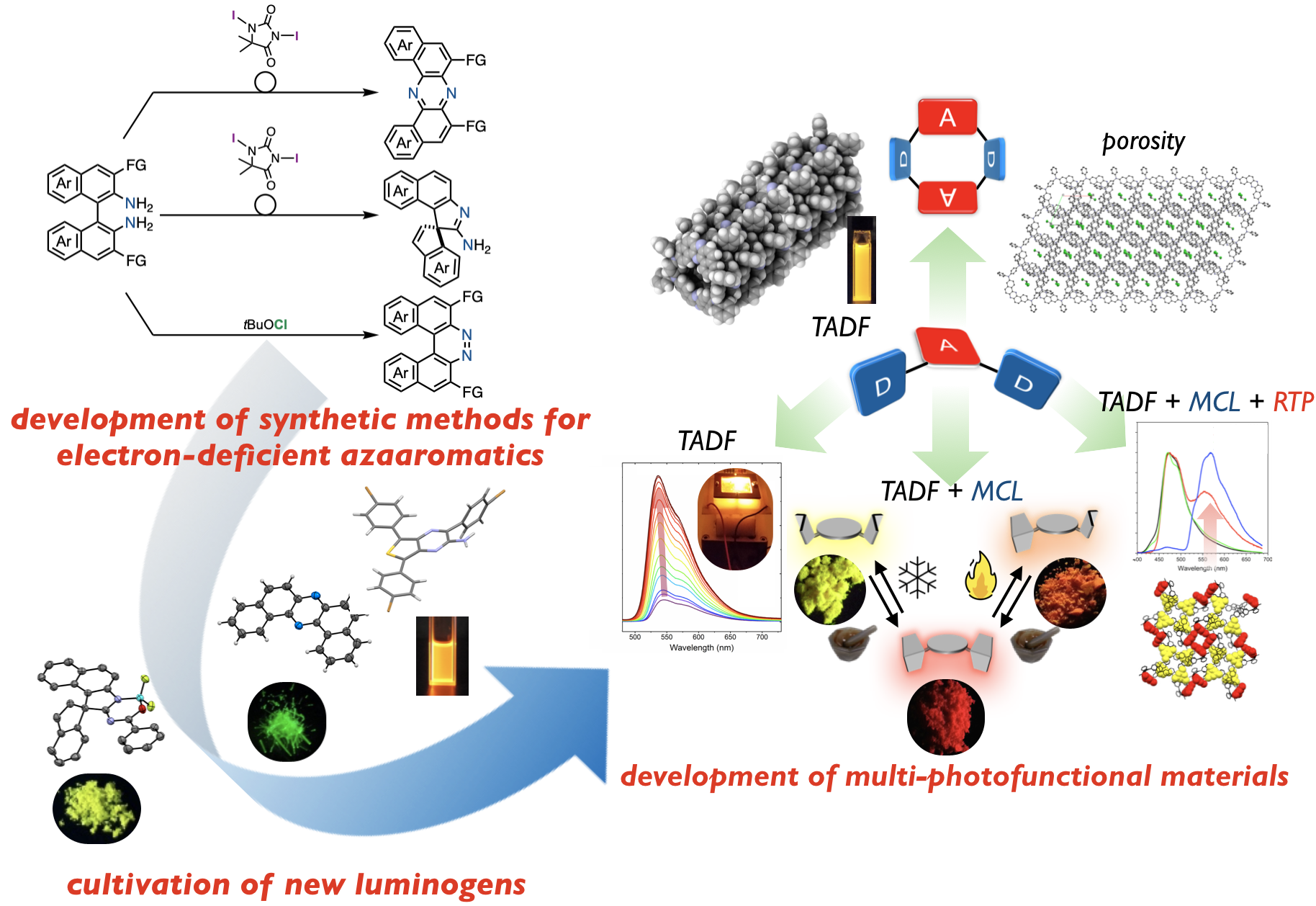
“Alchemy of Donor–Acceptor–Donor Multi-Photofunctional Organic Materials: From Construction of Electron-Deficient Azaaromatics to Exploration of Functions"
Youhei Takeda*, Przemyslaw Data*, and Satoshi Minakata
Chem. Commun. 2020, 56, 8884–8894. DOI:10.1039/D0CC03322G
*Open-access Article!
*Feature Article!
*Selected as the "Outside Front Cover" of the issue!

Artwork designed by Hayanon Science Manga Studio (2020)
Abstract: Electron-deficient azaaromatics play crucial roles in organic material fields. Therefore, the development of synthetic methods for electron-deficient azaaromatics and the exploration of their properties and functions is important for the advancement of materials sciences and related research fields. In this Feature Article, we describe new synthetic methods for exotic electron-deficient azaaromatics and their utilization in the design of multi-photofunctional organic materials. The key findings involve a novel oxidative skeletal rearrangement of binaphthaenediamines to give U-shaped azaaromics, i.e., dibenzo[a,j]phenazine, in good yields. The unique physicochemical features of the dibenzophenazine allow for the development of multi-photofunctional organic materials based on a U-shaped and twisted electron-donor–acceptor-donor scaffold. The developed compounds exhibit efficient thermally activated delayed fluorescence, mechanochromic luminescence, and room-temperature phosphorescence, and they serve as emissive materials in organic light-emitting diodes.
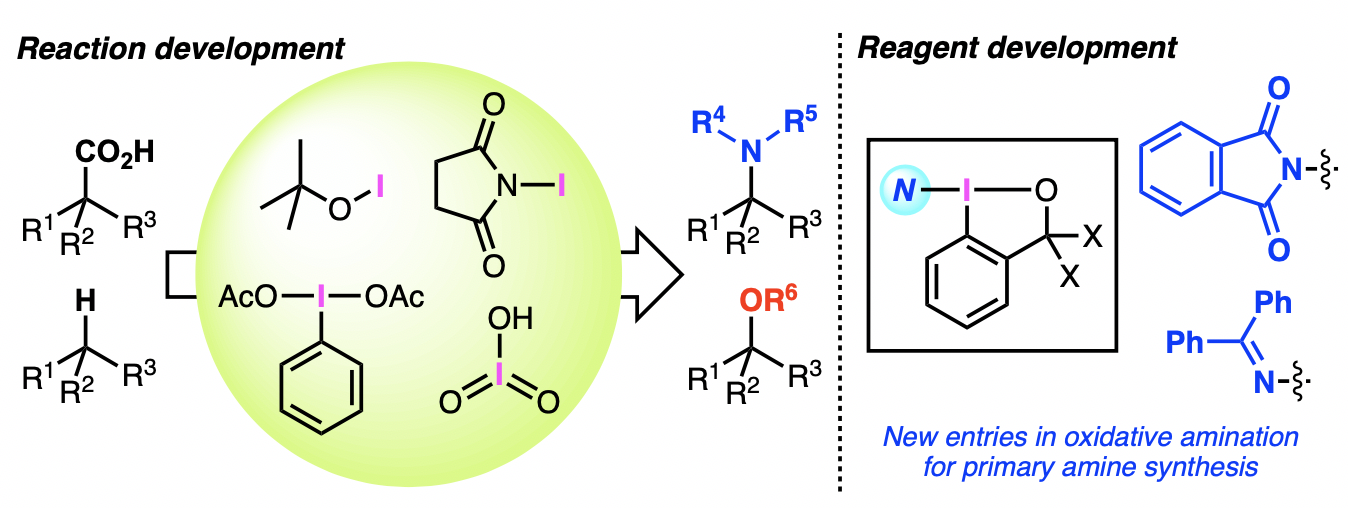
“Iodine-Based Reagents in Oxidative Amination and Oxygenation"
Kensuke Kiyokawa* and Satoshi Minakata*
Synlett 2020, 31, 845–855. DOI:10.1055/s-0039-1690827
*Account Article!
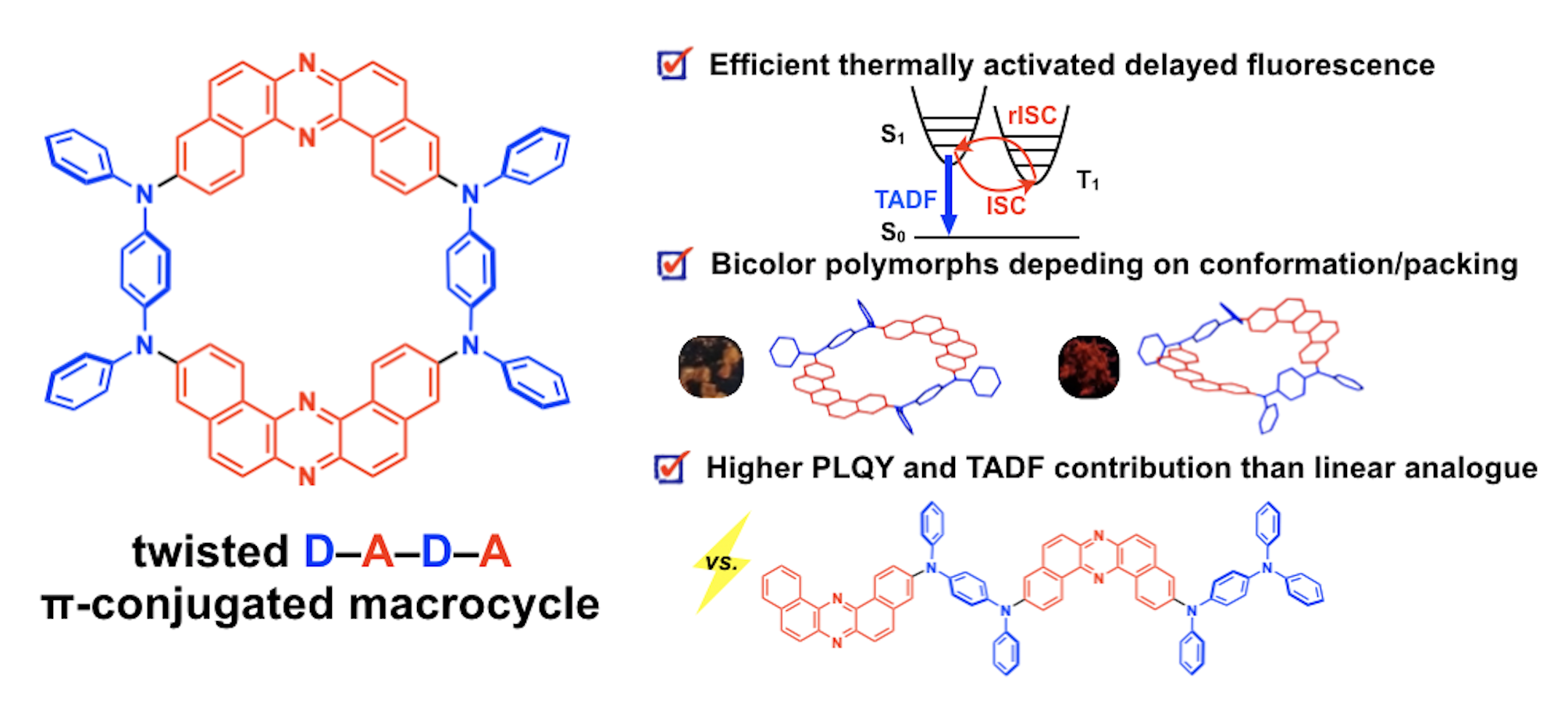
“Thermally Activated Delayed Fluorescent Donor–Acceptor–Donor–Acceptor π-Conjugated Macrocycle for Organic Light-Emitting Diodes"
Saika Izumi, Heather F. Higginbotham, Aleksandra Nyga, Patrycja Stachelek, Norimitsu Tohnai, Piotr de Silva, Przemyslaw Data*, Youhei Takeda*, and Satoshi Minakata*
J. Am. Chem. Soc. 2020, 142, 1482–1491. DOI:10.1021/jacs.9b11578
*Open-access Article!
*Highlighted in ResOU, Optronics, fabcross for エンジニア, EurekAlert!, AlphaGalileo, Phys.Org., Nanowerk, EE Times Japan, SienceDaily, Bioengineer.org, BITS&CHIPS, BrightSurt.com, BrightSurt.com, SciFi Insight!