2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
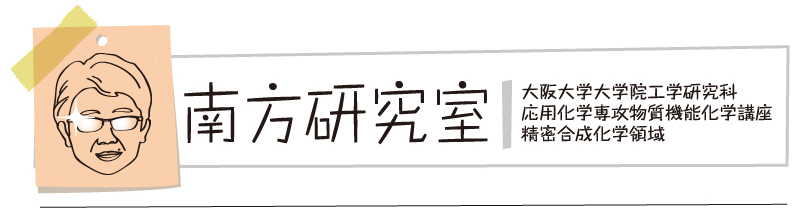
“Revealing the Internal Heavy Chalcogen Atom Effect on the Photophysics of the Dibenzo[a,j]phenazine-Cored Donor–Acceptor–Donor Triad"
Shimpei Goto, Yuya Nitta, Nicolas Oliveira Decarli, Leonardo Evaristo de Sousa, Patrycja Stachelek, Norimitsu Tohnai, Satoshi Minakata, Piotr de Silva*, Przemyslaw Data*, and Youhei Takeda*
J. Mater. Chem. C 2021, 9, 13942–13953. DOI:10.1039/D1TC02635F
pre-print version: ChemRxiv DOI:10.26434/chemrxiv.14401301.v1
*Selected as a part of the themed collection "2021 Journal of Materials Chemistry C Most Popular Articles"!
“Intramolecular C−H Amination of N-Alkylsulfamides by tert-Butyl Hypoiodite or N-Iodosuccinimide"
Kensuke Kiyokawa,* Keisuke Jou, and Satoshi Minakata*
Chem. Eur. J. 2021, Early View. DOI:10.1002/chem.202102635
*Selected as a HOT Paper!

Abstract: 1,3-Diamines are an important class of compounds that are broadly found in natural products and are also widely used as building blocks in organic synthesis. Although the intramolecular C−H amination of N-alkylsulfamide derivatives is a reliable method for the construction of 1,3-diamine structures, the majority of these methods involve the use of a transition-metal catalyst. We herein report on a new transition-metal-free method using tert-butyl hypoiodite (t-BuOI) or N-iodosuccinimide (NIS), enabling secondary non-benzylic and tertiary C−H amination reactions to proceed. The cyclic sulfamide products can be easily transformed into 1,3-diamines. Mechanistic investigations revealed that amination reactions using t-BuOI or NIS each proceed via different pathways.
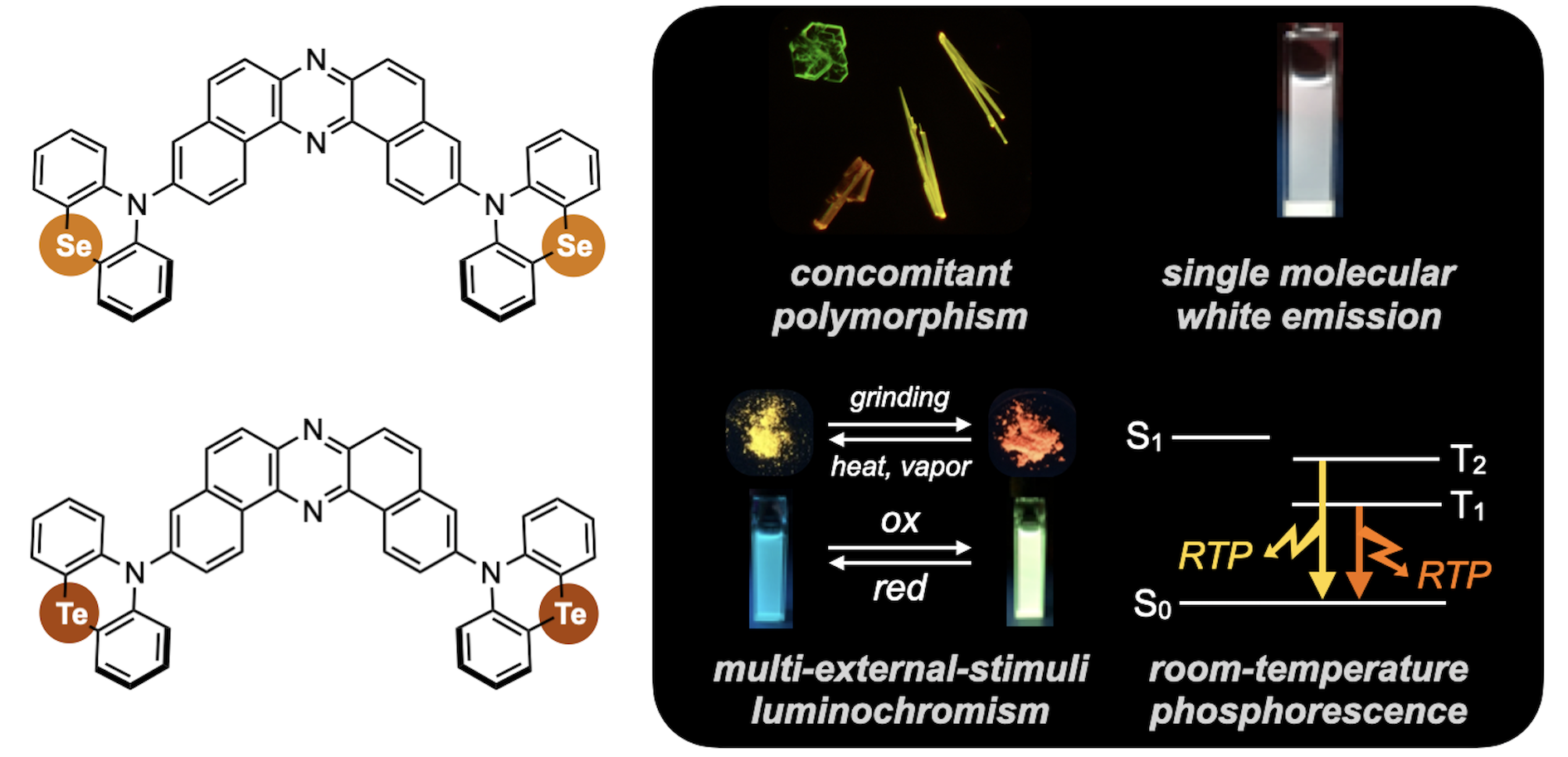
“The Impact of C2 Insertion into a Carbazole Donor on the Physicochemical Properties of Dibenzo[a,j]phenazine-Cored Donor–Acceptor–Donor Triads"
Paola Zimmermann Crocomo, Takahito Kaihara, Soki Kawaguchi, Patrycja Stachelek, Satoshi Minakata, Piotr de Silva*, Przemyslaw Data*, and Youhei Takeda*
Chem. Eur. J. 2021, 27, 13390–13398. DOI:10.1002/chem.202101654
*Open-access Article!
*Selected as a HOT Paper!
“A Practical Method for the Aziridination of α,β-Unsaturated Carbonyl Compounds with a Simple Carbamate Utilizing Sodium Hypochlorite Pentahydrate"
Takehiro Umeda and Satoshi Minakata*
RSC Adv. 2021, 11, 22120–22124. DOI:10.1039/D1RA04297A
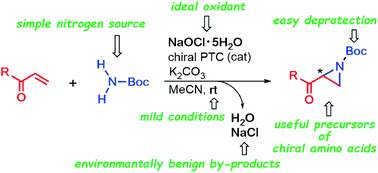
Abstract: The efficient formation of tert-butyl N-chloro-N-sodio-carbamate by the reaction of simple tert-butyl carbamate with sodium hypochlorite pentahydrate (NaOCl·5H2O) would be a practical and green method for the aziridination of α,β-unsaturated carbonyl compounds. The process described herein is transition-metal free, all of the materials are commercially available, the byproducts (NaCl and H2O) are environmentally benign and the reaction is stereoselective. The resulting aziridines are potential precursors of amino acids.
“Tris(pentafluorophenyl)borane-Catalyzed Formal Cyanoalkylation of Indoles with Cyanohydrins"
Kensuke Kiyokawa*, Naruyo Urashima, and Satoshi Minakata*
J. Org. Chem. 2021, 86, 8389–8401. DOI:10.1021/acs.joc.1c00808
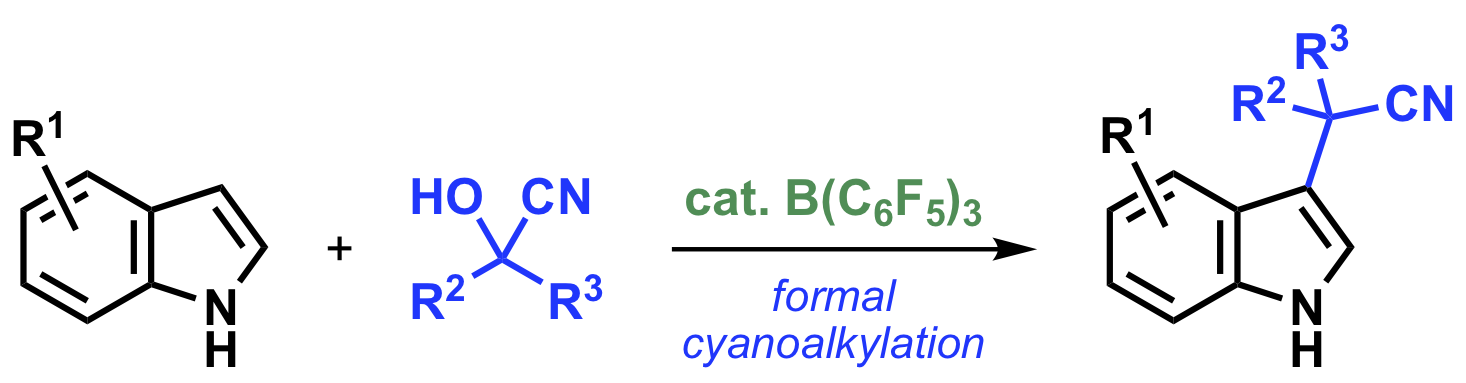
Abstract: We herein report on the formal C3-cyanoalkylation of indoles with cyanohydrins in the presence of a tris(pentafluorophenyl)borane B(C6F5)3 catalyst. It is noteworthy that cyanohydrins are used as a cyanoalkylating reagent in the present reaction even though they are usually used as only a HCN source. Mechanistic investigations revealed the unique reactivity of the B(C6F5)3 catalyst in promoting the decomposition of a cyanohydrin by a Lewis acidic activation through the coordination of the cyano group to the boron center. In addition, a catalytic three-component reaction using indoles, aldehydes as a carbon unit, and acetone cyanohydrin that avoids the discrete preparation of each aldehyde-derived cyanohydrin is also reported. The developed methods provide straightforward, highly efficient, and atom-economical access to various types of synthetically useful indole-3-acetonitrile derivatives containing α-tertiary or quaternary carbon centers.
“Near Fermi Superatom State Stabilized by Surface State Resonances in a Multiporous Molecular Network"
Shigeki Kawai*, Mohammad A. Kher-Elden, Ali Sadeghi, Zakaria M. Abd El-Fattah, Kewei Sun, Saika Izumi, Satoshi Minakata, Youhei Takeda*, and Jorge Lobo-Checa*
Nano Lett. 2021, 21, 6456–6462. DOI:10.1021/acs.nanolett.1c01200
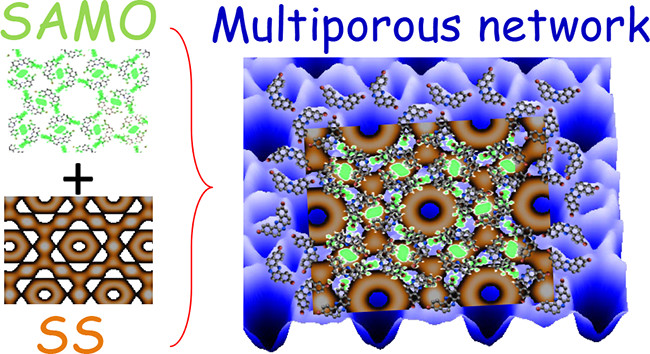
*Selected as the "Inside Cover" of the issue!

“Synthesis of Fused Diaziridine Derivatives from Cyclic Secondary Amines by Utilizing N-Bromosulfonamides as an Aminating Reagent"
Yuuki Kiyosu, Shino Tanaka, Sota Okumura, Kensuke Kiyokawa, and Satoshi Minakata*
Synthesis 2021, 53, 3103–3109. DOI:10.1055/a-1468-8275
*Published as part of the special topic "Bond Activation – in Honor of Prof. Shinji Murai"!
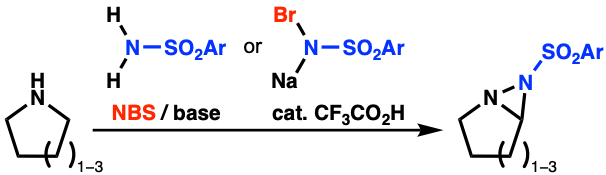
Abstract: The synthesis of a series of fused diaziridines, which are difficult to access by existing methods, was achieved by the reaction of cyclic secondary amines with sulfonamides in the presence of N-bromosuccinimide (NBS) and a suitable base. This oxidation system enables the efficient in situ formation of the key intermediates, which are N-bromoamines (a precursor of cyclic imines) and N-bromosulfonamides. In addition, an alternative method using bromamine salts in the presence of a catalytic amount of CF3CO2H for the synthesis of fused diaziridines is also reported.
“Palladium‐Catalyzed Regioselective and Stereospecific Ring‐Opening Suzuki‐Miyaura Arylative Cross‐Coupling of 2‐Arylazetidines with Arylboronic Acids"
Youhei Takeda*, Kazuya Toyoda, W. M. C. Sameera*, Norimitsu Tohnai, and Satoshi Minakata*
Adv. Synth. Catal. 2021, 363, 2796–2805. DOI:10.1002/adsc.202100195
*Highlighted in Hot Topic: C–C Coupling!
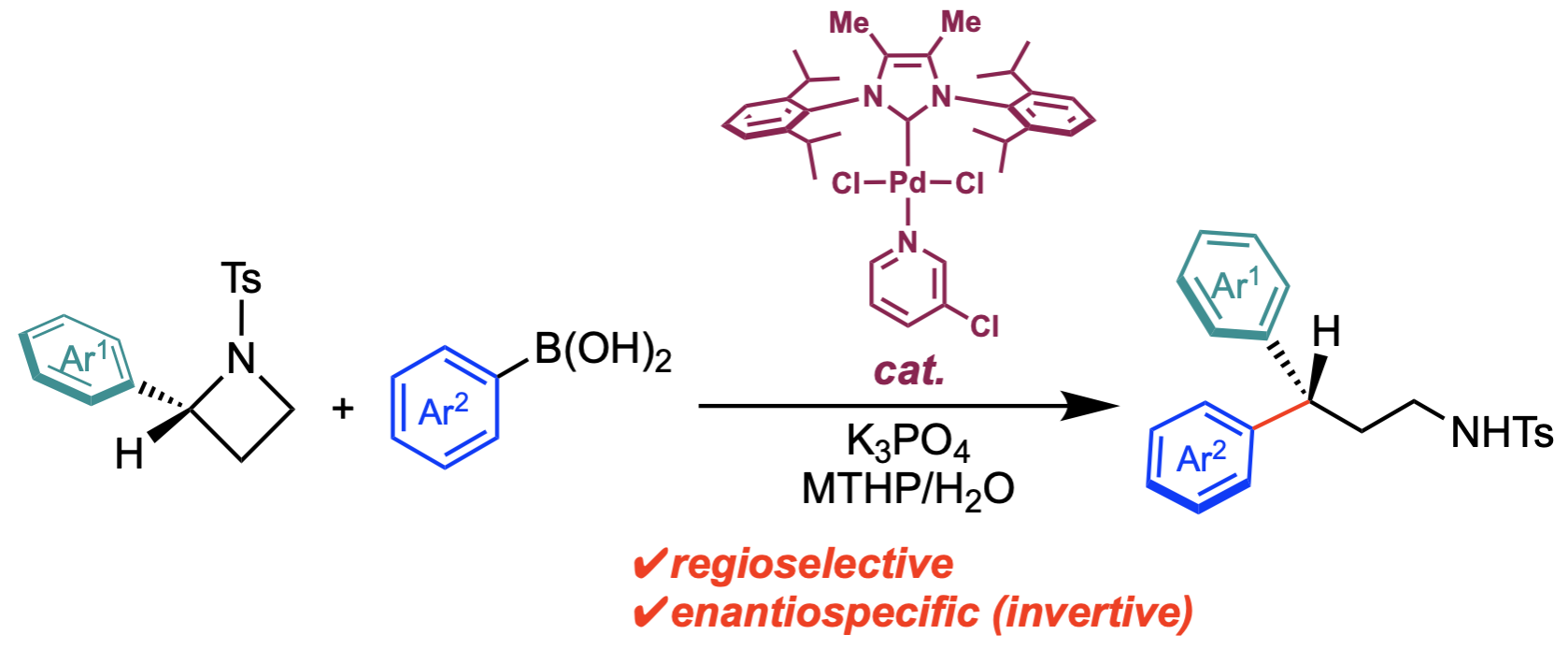
Abstract: We have developed a palladium‐catalyzed regioselective and enantiospecific ring‐opening Suzuki–Miyaura arylative cross‐coupling of N‐tosyl‐2‐arylazetidines to give enantioenriched 3,3‐diarylpropylamines. This reaction represents an example of transition‐metal‐catalyzed ring‐opening cross‐coupling using azetidines as a non‐classical alkyl electrophile. Density functional theory rationalized the mechanism of the full catalytic cycle, which consists of the selectivity‐determining ring opening of the azetidine, reaction with water, rate‐determining transmetalation, and reductive elimination. Transition states of the selectivity‐determining ring‐opening step were systematically determined by the multi‐component artificial force induced reaction (MC‐AFIR) method to explain the regioselectivity of the reaction.
“Diastereodivergent Intermolecular 1,2-Diamination of Unactivated Alkenes Enabled by Iodine Catalysis"
Satoshi Minakata*, Hayato Miwa, Kenya Yamamoto, Arata Hirayama, and Sota Okumura
J. Am. Chem. Soc. 2021, 143, 4112–4118. DOI:10.1021/jacs.1c00228
*Press Release
*Highlighted in Resou, EurekAlert!, AlphaGalileo,Bioengineer.org, PhysOrg, Florida News Times, Koliasa>/a>, Symbolic Resources Analytics! *Ranked in the Most Read Articles in April!
*Highlighted in SYNFORM! Synform 2021, 9, A141–A143. DOI:10.1055/s-0040-1720534.
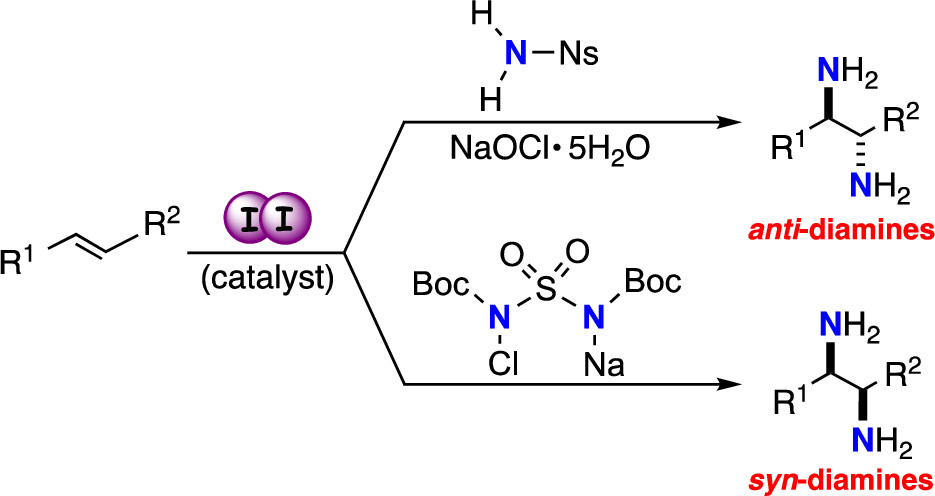
Abstract: The stereospecific, substrate (nitrogen source)-controlled intermolecular anti- and syn-1,2-diaminations of unactivated alkenes using the same catalysis (an iodine catalyst) is reported. The combined use of the two potential methods provides access to all of the disastereomeric forms of 1,2-diamines in spite of the availability of E- and Z-alkenes, and the resulting products can be readily converted into free vicinal diamines.
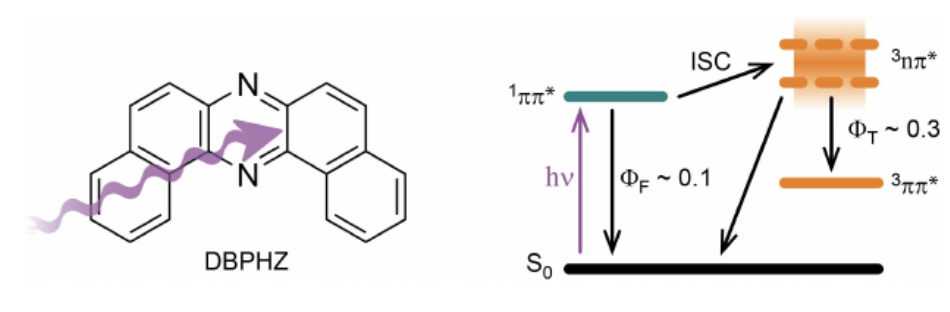
“The Photophysics of Dibenzo[a,j]phenazine"
Kristoffer A. Thom, Tom Förster, Oliver Weingart*, Shimpei Goto, Youhei Takeda*, Satoshi Minakata, and Peter Gilch*
ChemPhotoChem 2021, 5, 335–347. DOI:10.1002/cptc.202000250
*Open-access Article!
*Selected as the "Front Cover" of the issue!
*Highlighted in a Cover Profile! ChemPhotoChem 2021, 5, 297. DOI: 10.1002/cptc.202100065.

Artwork designed by Pauline Morys
Abstract: The photophysics of dibenzo[a,j]phenazine (DBPHZ) in solutionwas investigated by steady-state and time-resolved spectro-scopy. The nature of the solvent (methanol, acetonitrile,dichloromethane and cyclohexane were considered) onlyweakly affects the photophysical properties. The first excitedsinglet state features a lifetime of the order of 1 ns. Itsfluorescence emission is centered around 420 nm and occurswith moderate yields of ~0.1. The lowest triplet state ispopulated with yields of ~0.3. Quantum chemical computa-tions suggest that a 3nπ*state energetically close to theprimarily excited singlet state is responsible for the efficientintersystem crossing towards the triplet manifold“Heavy-Atom-Free Room-Temperature Phosphorescent Organic Light-Emitting Diodes Enabled by Excited States Engineering"
Heather F. Higginbotham, Masato Okazaki, Piotr de Silva*, Satoshi Minakata, Youhei Takeda*, and Przemyslaw Data*
ACS Appl. Mater. Interfaces 2021, 13, 2899–2907. DOI:10.1021/acsami.0c17295
pre-print version: ChemRxiv DOI:10.26434/chemrxiv.12818837.v1
*Press Release
*Highlighted in ResOU, Optronics, fabcross for エンジニア, EurekAlert!, AlphaGalileo, PhysOrg, Nanowerk, Bioengineering.org, SciFic Insight, Innovation News Network, Scienmag, Australian Online News!
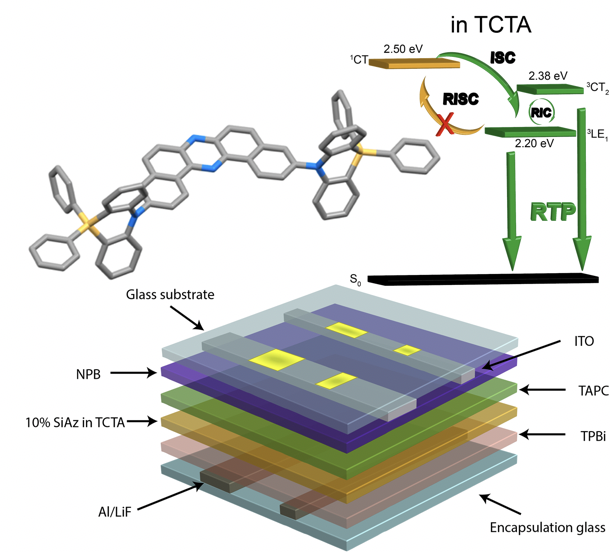
Abstract: Room temperature phosphorescence materials offer great opportunities for applications in optoelectronics, due to their unique photophysical characteristics. However, heavy-atom-free organic emitters that can realize distinct electrophosphorescence are rarely exploited. Herein a new approach for designing heavy-atom-free organic room temperature phosphorescence emitters for organic light-emitting diodes is presented. The subtle tuning of the singlet and triplet excited states energies by appropriate choice of host matrix allows tailored emission properties and switching of emission channels between thermally activated delayed fluorescence and room temperature phosphorescence. Moreover, an efficient and heavy-atom-free room temperature phosphorescence organic light-emitting diode using the developed emitter is realized.
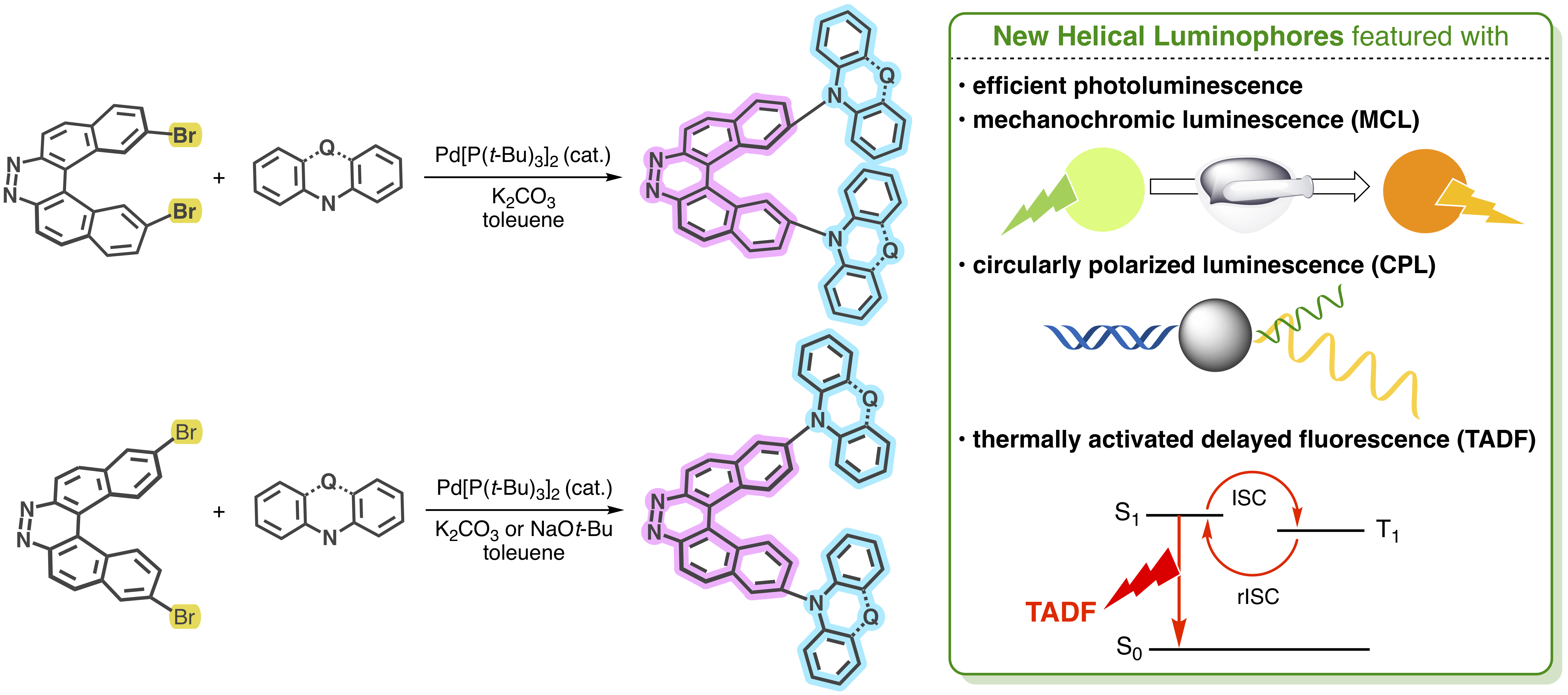
“Peripherally Donor-Installed 7,8-Diaza[5]helicenes as a Platform for Helical Luminophores"
Yuta Ikari, Takahito Kaihara, Shimpei Goto, Marcel Bovenkerk, David C. Grenz, Birgit Esser∗, Marli Ferreira, Patrycja Stachelek, Przemyslaw Data∗, Takumu Yoshida, Tomoyuki Ikai, Norimitsu Tohnai, Satoshi Minakata, and Youhei Takeda∗
Synthesis 2021, 53, 1584–1596. DOI:10.1055/a-1343-5810
*Invited as a Feature Article!
*Selected as the "Front Cover" of the issue!