home > Pablication
2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
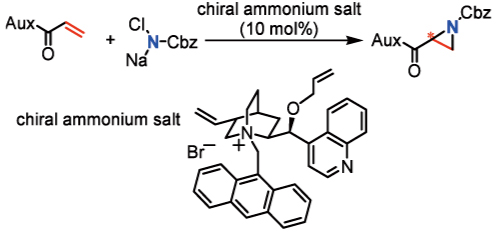
"Catalytic Aziridination of Electron-Deficient Olefins with N-Chloro-N-sodiocarbamate and Application of This Novel Method to Asymmetric Synthesis"
Satoshi Minakata,* Yuta Murakami, Ryoji Tsuruoka, Shinsuke Kitanaka, and Mitsuo Komatsu
Chem. Commun. 2008, 6363-6365. DOI: 10.1039/b812978a
"Inclusion of C60 into MCM-41 by Solvophobic Nature"
Satoshi Minakata,* Ryoji Tsuruoka, and Mitsuo Komatsu
J. Am. Chem. Soc. 2008, 130, 1536-1537. DOI: 10.1021/ja077114w

"Ionic Introduction of an N1 Unit to C60 and Unique Rearrangement of Aziridinofullerene"
Satoshi Minakata,* Ryoji Tsuruoka, Toshiki Nagamachi, and Mitsuo Komatsu
Chem. Commun. 2008, 323-325. DOI: 10.1039/b715348a
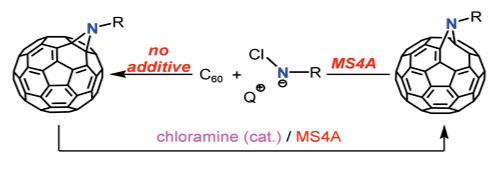
Abstract: A new chloramine-based aziridination of C60 and unique rearrangement of aziridinofullerene to azafulleroid is described. The ionic introduction of an N1 unit to C60 via an addition–cyclization mechanism was first achieved under mild conditions; the combination of chloramine and MS4A resulted in the promising rearrangement of aziridinofullerene to azafulleroid, and the isomerization could be performed catalytically.
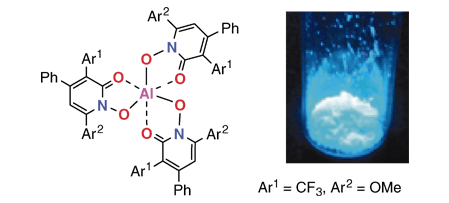
"Novel Fluorescent Aluminum ComplexesBased on N-Hydroxy-3,6-diaryl-4-phenyl-2-pyridone Ligands"
Satoshi Minakata,* Hiroshi Inada, Mitsuo Komatsu, Hirotake Kajii, Yutaka Ohmori,* Manabu Tsumura, and Kiyoyuki Namura
Chem Lett. 2008, 37, 248-249. DOI:10.1246/cl.2008.248