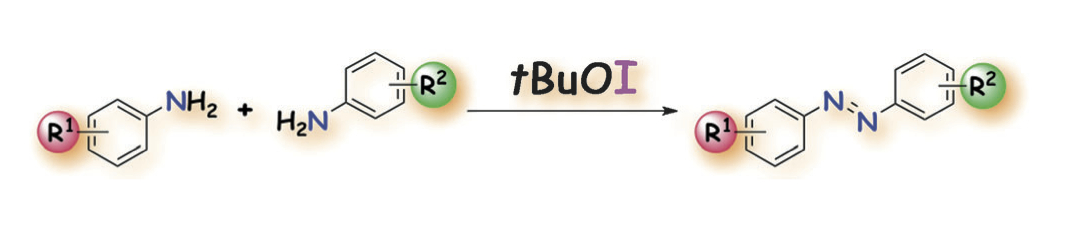home > Pablication
2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
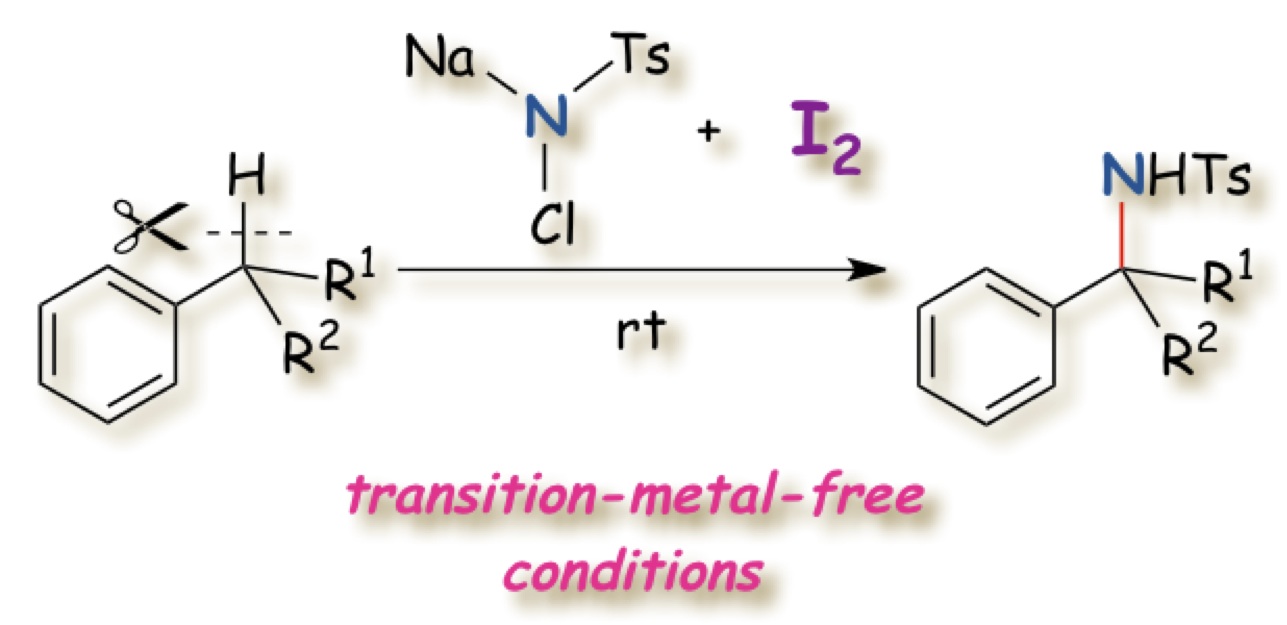
"Transition-metal-free Benzylic C–H Bond Intermolecular Amination Utilizing Chloramine-T and I2"
Youhei Takeda, Junpei Hayakawa, Kazuki Yano, and Satoshi Minakata*
Chem. Lett. 2012, 41, 1672–1674. DOI: 10.12461/cl.2012.1672
"Nucleophilic Ring-Opening of N-o-Nosylaziridines with N-Chloro-N-Sodiocarbamate: Facile Preparation of Differentially Protected Vicinal Diamines"
Youhei Takeda, Yuta Murakami, Yuki Ikeda, and Satoshi Minakata*
Asian J. Org. Chem. 2012, 1, 226–230. DOI: 10.1021/ajoc.201200070
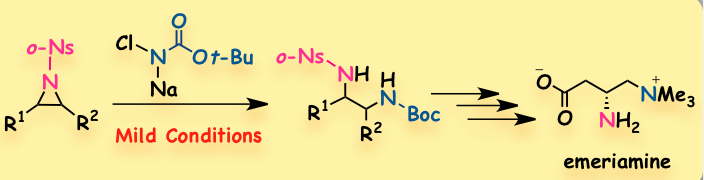
Abstract: A mild nucleophilic ring-opening reaction of activated aziridines with N-chloro-N-sodio-tert-butylcarbamate, which gives differentially protected vicinal diamine derivatives, has been developed. The two protecting groups are selectively removable, and the total synthesis of a long-chain fatty acids oxidation inhibitor, emeriamine, was achieved by using the present method.
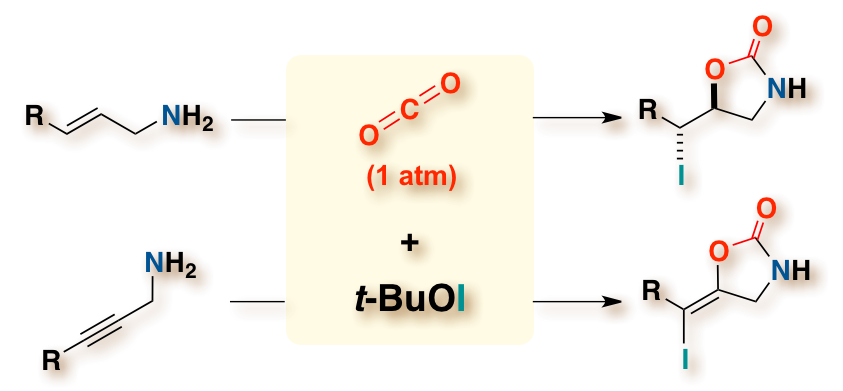
"Cyclizative Atmospheric CO2 Fixation by Unsaturated Amines with t-BuOI Leading to Cyclic Carbamates"
Youhei Takeda, Sota Okumura, Saori Tone, Itsuro Sasaki, and Satoshi Minakata*
Org. Lett. 2012, 14, 4874–4877. DOI: 10.1021/ol302201q
* Highlighted in "Noteworthy Chemistry" (ACS, October 1, 2012)! (see the detail)
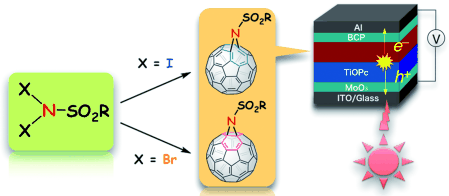
"Selective Functionalization of Fullerenes with N,N-Dihalosulfonamides as an N1 Unit: Versatile Syntheses of Aza[60]fulleroids and Aziridino[60]fullerenes and their Application to Photovoltaic Cells"
Toshiki Nagamachi, Youhei Takeda, Kazuhisa Nakayama, and Satoshi Minakata*
Chem. Eur. J. 2012, 18, 12035-12045. DOI: 10.1002/chem.201201680
"Oxidative Dimerization of Aromatic Amines Using tBuOI under Mild Conditions: Entry to Unsymmetric Aromatic Azo Compounds"
Youhei Takeda, Sota Okumura, and Satoshi Minakata*
Angew. Chem., Int. Ed. 2012, 51, 7804-7808. DOI: 10.1002/anie.201202786
* Selected as a "Hot Paper"! (see the detail)
* Highlighted in "ワイリー・サイエンスカフェ"! (link)
* Highlighted in "Noteworthy Chemistry" ACS, August 27, 2012! (see the detail)
* Highlighted in Synfacts (2012, 8, 1091)! (see the detail)