2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
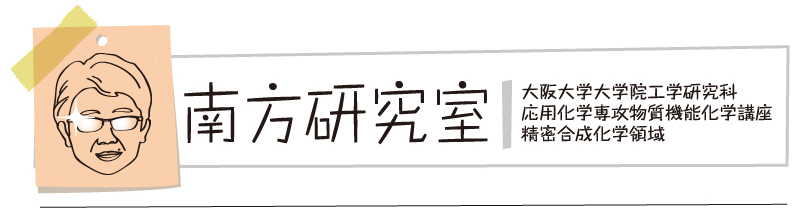
"Catalytic Activation of 1-Cyano-3,3-dimethyl-3-(1H)-1,2-benziodoxole with B(C6F5)3 Enabling the Electrophilic Cyanation of Silyl Enol Ethers"
Takaya Nagata, Hiroki Matsubara, Kensuke Kiyokawa*, and Satoshi Minakata*
Org. Lett. 2017, 19, 4672–4675. DOI:10.1021/acs.orglett.7b02313
"An Optical and Electrical Study of Full Thermally Activated Delayed Fluorescent White Organic Light-Emitting Diodes"
Daniel de Sa Pereira,* Paloma L. dos Santos, Jonathan S. Ward, Przemyslaw Data, Masato Okazaki, Youhei Takeda, Satoshi Minakata, Martin R. Bryce, and Andrew P. Monkman
Sci. Rep. 2017, 7, 6234/1-8. DOI:10.1038/s41598-017-06568-3
*Open-access Article!
Abstract: We report on the engineering of full thermally activated delayed fluorescence – based white organic light emitting diodes (W-OLEDs) composed of three emitters (2,7-bis(9,9-dimethyl-acridin-10-yl)-9,9-dimethylthioxanthene-S,S-dioxide (DDMA-TXO2), 2,7-bis(phenoxazin-10-yl)-9,9-dimethylthioxanthene-S,S-dioxide (DPO-TXO2) and 3,11-di(10H-phenoxazin-10-yl)dibenzo[a,j]phenazine (POZ-DBPHZ) in two different hosts. By controlling the device design through the study of the emission of DDMA-TXO2 and DPO-TXO2, the behaviour of POZ-DBPHZ in a device with more than one emitter, and the combination of the three materials, respectively, we show that external quantum efficiencies as high as 16% can be obtained for a structure with a correlated colour temperature close to warm white, together with colour rendering index close to 80. However it is in their performance stability that provides the true breakthrough: at 1000 cd/m2 the efficiencies were still above 10%, which is one of the best for this type of devices.
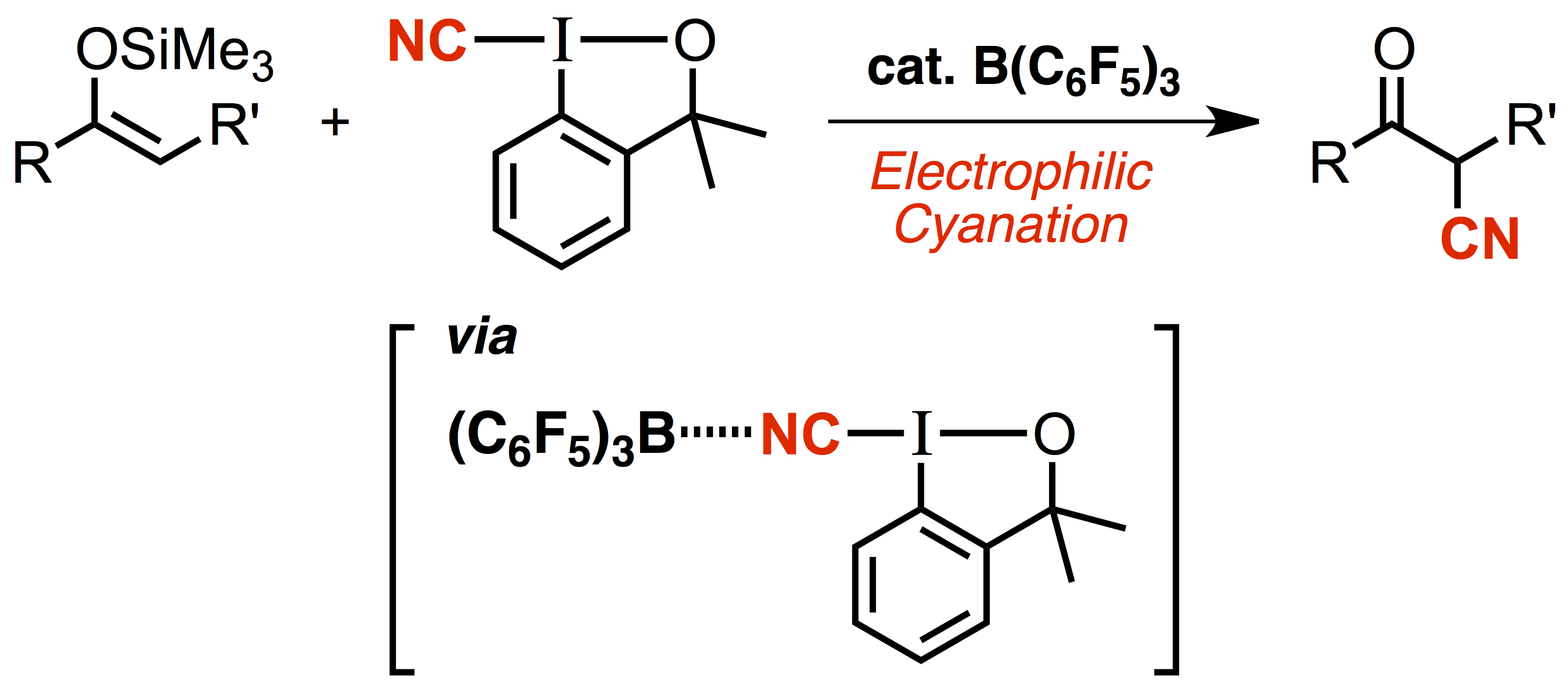
"Hypervalent Iodine(III)-Mediated Decarboxylative Ritter-Type Amination Leading to the Production of α-Tertiary Amine Derivatives"
Kensuke Kiyokawa*, Tomoki Watanabe, Laura Fra, Takumi Kojima, and Satoshi Minakata*
J. Org. Chem. 2017, 82, 11711–11720. DOI:10.1021/acs.joc.7b01202
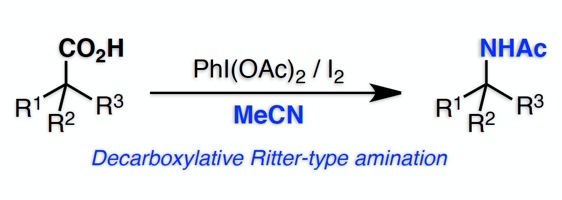
"Oxidative Cyclization of β,γ-Unsaturated Carboxylic Acids Using Hypervalent Iodine Reagents: An Efficient Synthesis of 4-Substituted Furan-2-ones"
Kensuke Kiyokawa*, Kenta Takemoto, Shunsuke Yahata, Takumi Kojima, and Satoshi Minakata*
Synthesis 2017, 49, 2907–2912. (Published as part of the Special Topic "Modern Strategies with Iodine in Synthesis") DOI:10.1055/s-0036-1588987
Abstract: The oxidative cyclization of β-substituted β,γ-unsaturated carboxylic acids using a hypervalent iodine reagent, to provide 4-substituted furan-2-one products, is reported. In this cyclization, the use of a highly electrophilic PhI(OTf)2, which is in-situ prepared from PhI(OAc)2 and Me3SiOTf, is crucial. Depending on the substitution pattern at the α-position of the substrates, furan-2(5H)-ones and furan-2(3H)-ones are produced. Thus, the present method offers a useful tool for accessing various types of 4-substituted furan-2-ones that are important structural motifs in the field of organic chemistry and medicinal chemistry.
"Thermally Activated Delayed Fluorescent Phenothiazine-Dibenzo[a,j]phenazine-Phenothiazine Triads Exhibiting Tricolor-Changing Mechanochromic Luminescence"
Masato Okazaki, Youhei Takeda*, Przemyslaw Data*, Piotr Pander, Heather Higginbotham, Andrew P. Monkman, and Satoshi Minakata*
Chem. Sci. 2017, 8, 2677–2686. DOI:10.1039/C6SC04863C
*Open-access Article!
*Highlighted in ResOU, Chem-Station, AlphaGalileo, EurekAlert!, Phys Org, ScienceDaily, and UPI, Asian Scientist, American Laboratory!
*Ranked as the "Most downloaded articles of 2017: Inorganic and Physical Chemistry"!(see the detail)
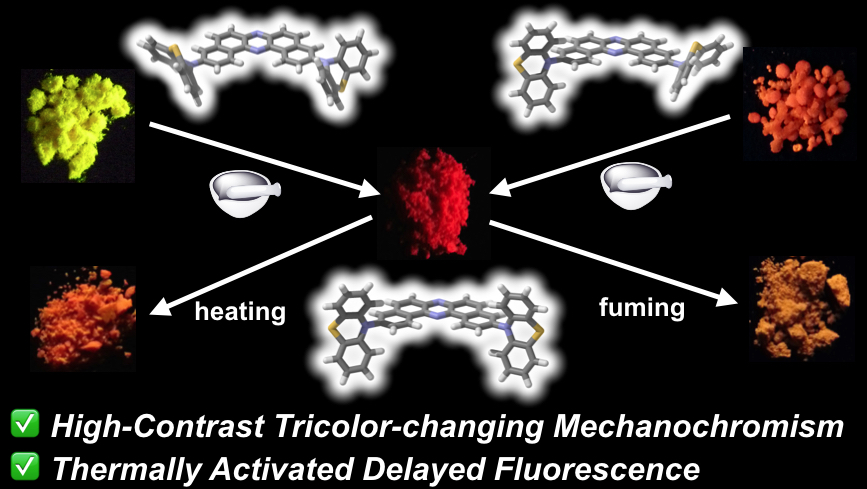
Abstract: Novel U-shaped donor-acceptor-donor (D-A-D) π-conjugated multi-functional molecules comprising of dibenzo[a,j]phenazine (DBPHZ) as an acceptor and phenothiazines (PTZ) as donors have been developed. Most importantly, the D-A-D compounds exhibit not only distinct tricolor-changeable mechanochromic luminescence (MCL) properties but also efficient thermally activated delayed fluorescence (TADF). Quantum chemical calculations, X-ray diffraction analysis, and systematic studies on photophysical properties have indicated that the “two-conformation-switchable” PTZ units play a highly important role in achieving multi-color-changing MCL. Time-resolved photophysical measurements have revealed that developed D-A-D compounds also exhibit efficient orange-TADF. Furthermore, organic light-emitting diode (OLED) devices fabricated with the new TADF emitters have achieved high external quantum efficiencies (EQEs) up to 16.8%, which significantly exceeds theoretical maximum (~5%) with conventional fluorescent emitters.
"Oxidative Self-annulation of 2,5-Diaryl-3,4-diaminothiophene via C–C and C–S Bond Cleavage of the Thiophene Ring: A New Synthesis of an Amino-substituted Triarylthieno[3,4-b]pyrazines and Their Photophysical Properties"
Youhei Takeda*, Satoshi Ueta, and Satoshi Minakata*
Heterocycles, 2017, 95, 137–144. (a Special Issue in honor of Professor Dr. Masakatsu Shibasaki on 70th Birthday) DOI:10.3987/COM-16-S(S)11
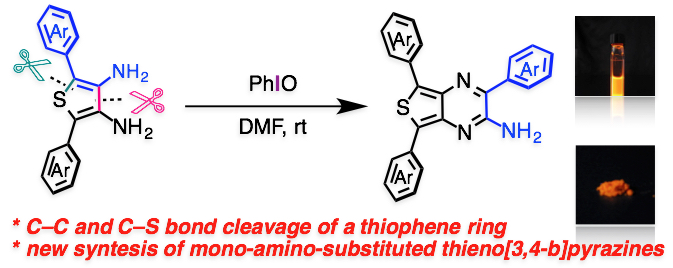
Abstract: A novel oxidative self-annulation of 2,5-diaryl-3,4-diaminothiophenes that accompanies C–C and C–S bonds cleavage of a thiophene ring to produce 3,5,7-triaryl-2-aminothieno[3,4-b]pyrazines in moderate to good yields has been discovered. Photophysical properties of this new family of thienopyrazines have also been disclosed.