2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
2015
2014
2013
2012
2011
2010
2009
2008
1998–2007
"Diastereoselective Aziridination of Chiral Electron-Deficient Olefins with N-Chloro-N-sodiocarbamates Catalyzed by Chiral Quaternary Ammonium Salts"
Yuta Murakami, Youhei Takeda, and Satoshi Minakata*
J. Org. Chem. 2011,
76, 6277-6285.
DOI: 10.1021/jo2010632
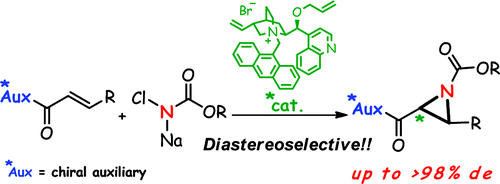
Abstract: Chiral quaternary ammonium salt-catalyzed diastereoselective aziridination of electron-deficient olefins that possess a chiral auxiliary with
N-chloro-
N-sodiocarbamates was developed. The key to high stereoselectivity was found to be the employment of the “matching” stereochemical combination of chiral auxiliary/ammonium salt. For example, when 3-phenyl-(4
R,7
S)-4-methyl-7-isopropyl-4,5,6,7-tetrahydroindazole (L-menthopyrazole) as a chiral auxiliary and a cinchonidine-derived chiral ammonium salt as a catalyst were applied to the reaction system, perfect diastereoselectivity was realized. Furthermore, the preparation of enantiomerically pure aziridines by removal of the chiral auxiliary was demonstrated.
"Generation of Nitrile Oxides from Oximes Using t-BuOI and Their Cycloaddition
Satoshi Minakata,* Sota Okumura, Toshiki Nagamachi, and Youhei Takeda
Org. Lett. 2011,
13, 2966-2969.
DOI: 10.1021/ol2010616
* Selected as one of the top 20 most read articles in June 2011!
(
see the detail)
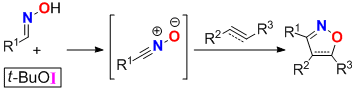
Abstract:
tert-Butyl hypoiodite (
t-BuOI) was found to be a powerful reagent for the cycloaddition of oximes and alkenes/alkynes, leading to the formation of a variety of isoxazolines or isoxazoles under mild conditions.
"The Diels-Alder reaction of C60 and cyclopentadiene in mesoporous silica as a reaction medium"
Satoshi Minakata,* Toshiki Nagamachi, Kazuhisa Nakayama, Takeyuki Suzuki, and Takanori Tanaka
Chem.Commun. 2011,
47, 6338-6340.
DOI: 10.1039/c1cc11437a
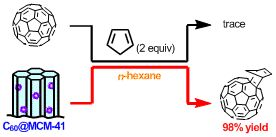
Abstract:As a new organic reaction medium, a periodic mesoporous inorganic material was found to function as a “solid solvent” in a Diels–Alder reaction of C
60 with cyclopentadiene. This finding was supported by a concentration effect and a kinetic study of the reaction.
"Iodoamidation of Olefins with Chloramine Salts and Iodine in Aqueous Media"
Satoshi Minakata* and Junpei Hayakawa
Chem.Commun. 2011,
47, 1905-1907.
DOI: 10.1039/C0CC03855E
* Selected as one of the top 10 most accessed articles in December 2010!
(see the detail)
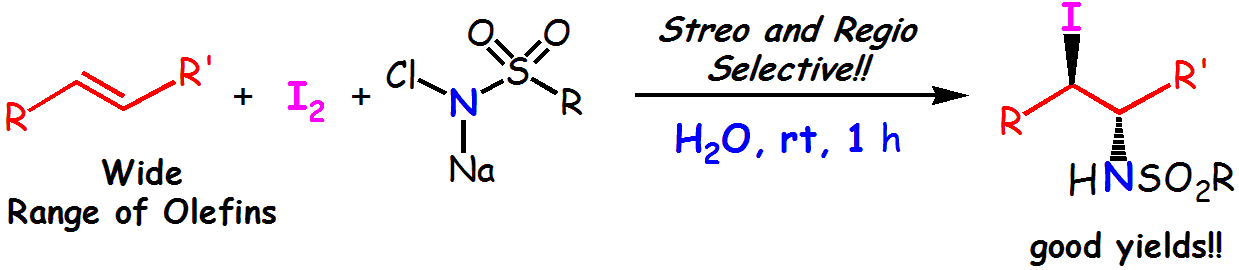
Abstract: An efficient, unique, and convenient method for the iodoamidation of olefins with chloramine salts and I
2 in aqueous media is described. This method was applicable to a wide range of olefins, including aromatic, aliphatic, and electron-deficient olefins.