An Isolated Lithium ortho-Carboranyl Cuprate Complex for the Synthesis of Multiple-Carborane-Substituted Arenes from (Hetero)Aryl Bromides and Chlorides
Y. Hisata, D. Morishita, Y. Hoshimoto
J. Am. Chem. Soc. 2025, 147, 37677-37687.
We report the isolation and characterization of a lithium bis(o-carboran-1-yl)cuprate complex (Li/Cu-1) that
enables the efficient “dump-and-stir” synthesis of
carborane-substituted arenes from readily available aryl bromides and
chlorides. Remarkably, isophthalonitrile functions as a ligand for the
Li center in Li/Cu-1, leaving the Cu center available for the oxidative addition to aryl halides.
OPEN ACCESS; Featured byAsia Research News; EurekAlert; PhysOrg; AlphaGalileo;


Reductive Preparation of Fluoroalkyl Copper Complexes from Perfluorinated Alkyl Iodides Using Diboron
H. Nishiguchi, S. Ogoshi, R. Doi
J. Org. Chem. 2025, 90, 13844-13848.
The reductive synthesis of fluoroalkyl copper phenanthroline complexes from fluoroalkyl iodides, using diboron as a reductant, is reported in this study. Furthermore, a one-pot protocol enabled the reductive coupling of aryl iodides and fluoroalkyl iodides without the need to isolate the intermediate fluoroalkyl copper complexes.

研究者はどこかクレイジーでなければいけない~Lectureship Award MBLA 2024 受賞講演ツアーを終えて~
星本 陽一
有機合成化学協会誌2025 年 83 巻 6 号 p. 551-562.
PDFはこちらから(許可取得済)
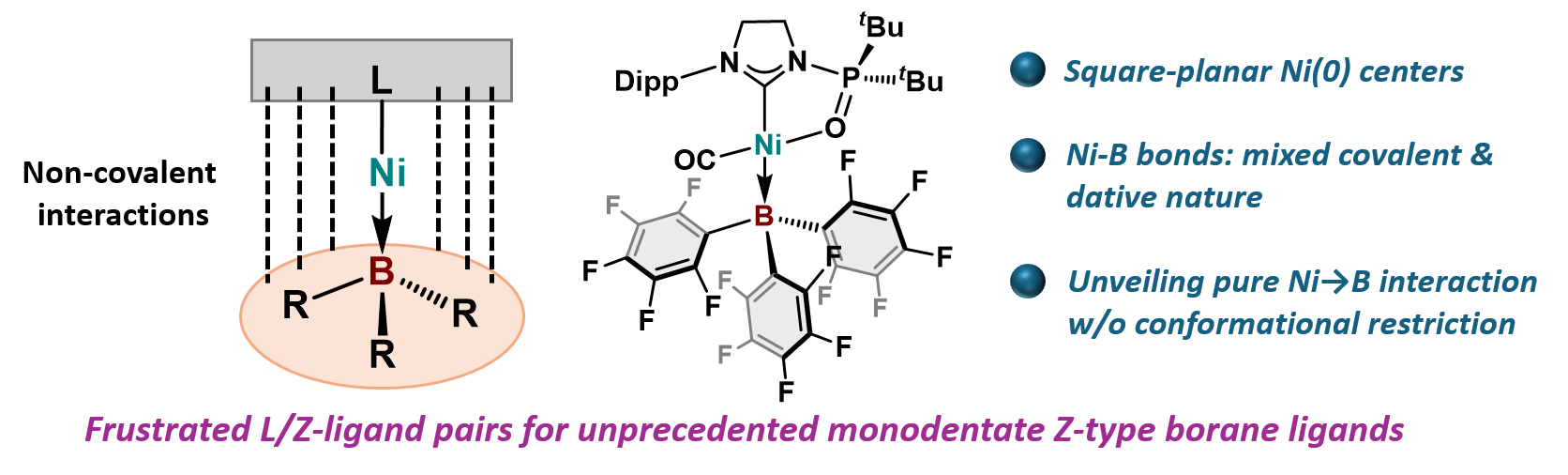
Monodentate σ-Accepting Boron-Based Ligands Bearing Square-Planar Ni(0) Centers
Y. Mondori, Y. Yamauchi, T. Kawakita, S. Ogoshi, Y. Uetake, Y. Takeichi,
H. Sakurai, Y. Hoshimoto
J. Am. Chem. Soc. 2025, 147, 8326-8335.
We report the synthesis and characterization of square-planar Ni(0) complexes
that bear tris(perfluoroaryl)boranes as monodentate Z-type ligands. A combined
theoretical and experimental approach revealed a mixed covalent/dative
character for the Ni–B bonds. This strategy uses frustrated L/Z-ligand
pairs that combine sterically encumbered electron-donating (L-type) and
electron-accepting ligands to form noncovalent interactions over L–M–Z
units to achieve unprecedented low-valent transition metal species with
monodentate Z-type ligands.
OPEN ACCESS; Featured by EurekAlert; Asia Research News; PhysOrg; AlphaGalileo;
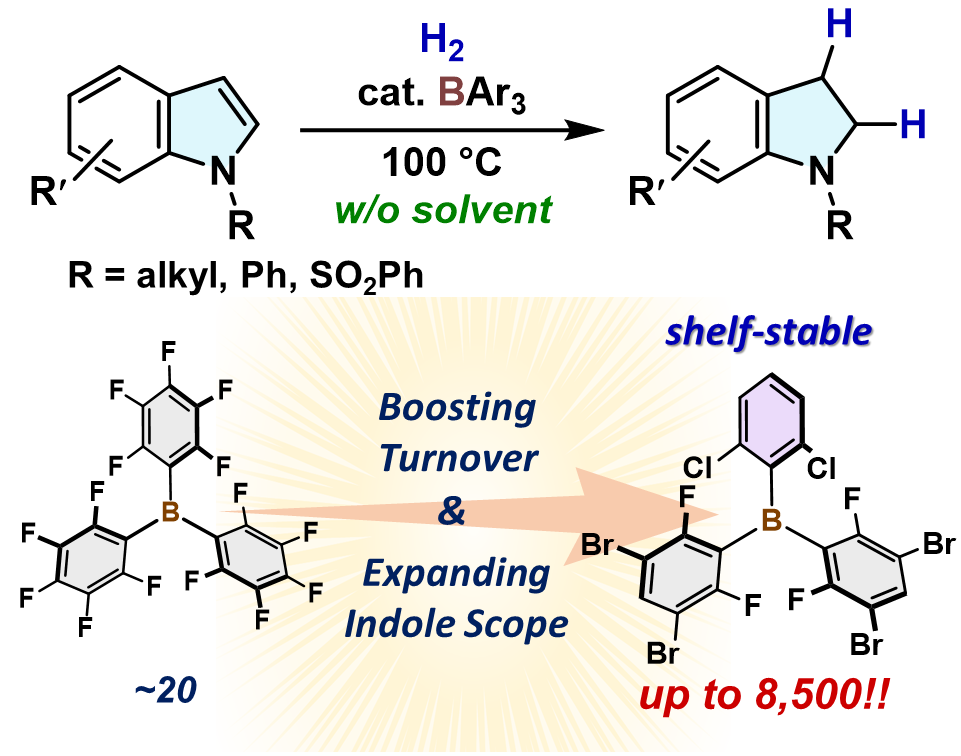
Boosting Turnover in the Triarylborane-Catalyzed Hydrogenation of N-Substituted Indoles via Olefin-to-Nitrogen Lewis Base Switching in H2-Cleavage Steps
T. Hashimoto, M. Tanigawa, K. Kambe, S. Ogoshi, Y. Hoshimoto
Precis. Chem. 2025, 3, 128-134.
The shelf-stable heteroleptic borane B(2,6-Cl2C6H3)(2,6-F2-3,5-Br2C6H)2 efficiently catalyzes the solvent-free hydrogenation of various substituted
indoles to indolines with an unprecedented turnover number of 8,500, which
is more than 400-fold higher than that reported for B(C6F5)3 under diluted conditions. This study demonstrates the potential of relatively
benign main-group elements for the catalytic synthesis of valuable N-containing molecules using
H2.
OPEN ACCESS
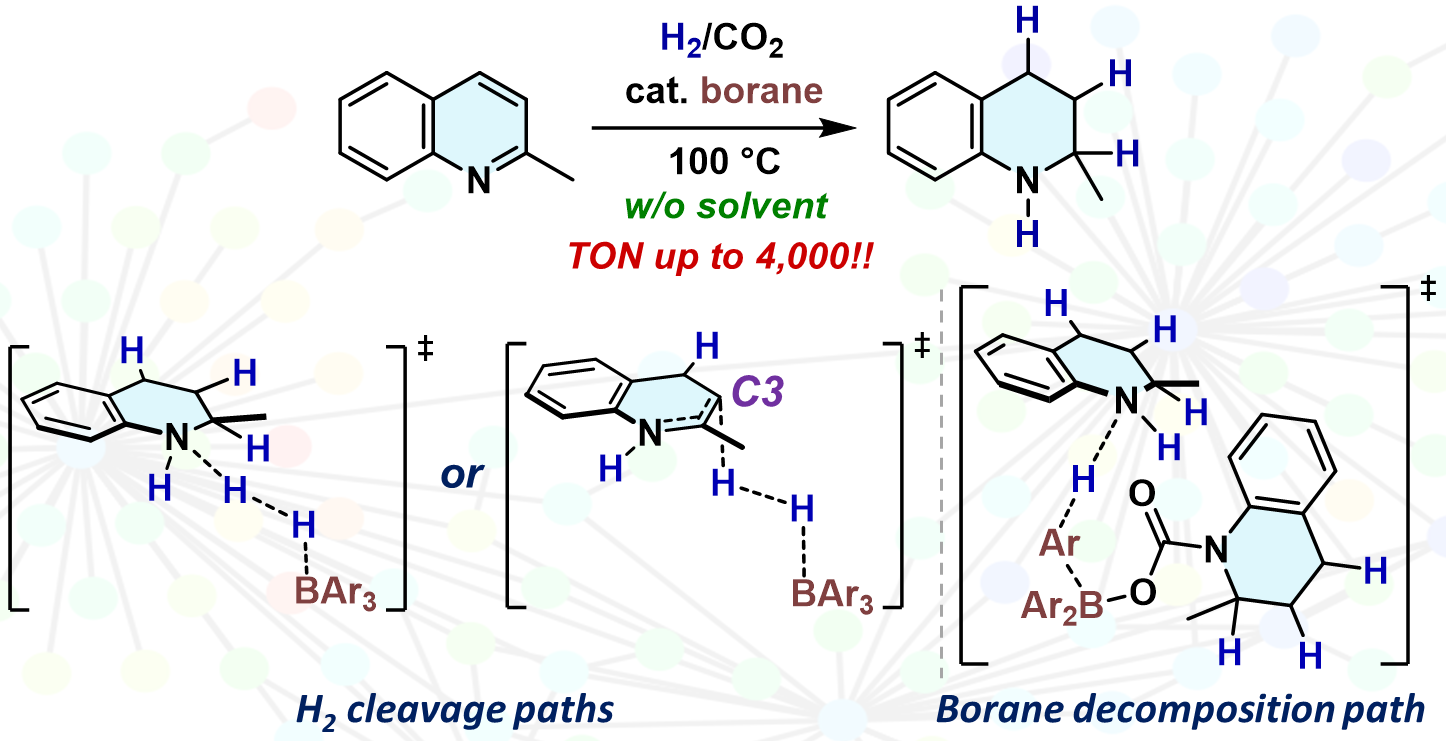
Elucidating multicomponent mechanisms in the catalytic hydrogenation of 2-methylquinoline under crude-H2 conditions: a key H2-cleavage process by a boron–olefin Lewis pair
T. Hashimoto, Yu Harabuchi, S. Ogoshi, S. Maeda, Y. Hoshimoto
Bull. Chem. Soc. Jpn., 2025, 98, uoae145.
The mechanisms of the triarylborane-catalyzed hydrogenation of 2-methylquinoline
(MeQin) in the presence of CO2 were investigated using the artificial force induced reaction method.
Based on these mechanistic details, we identified the modified catalyst
B(2-Cl-6-FC6H3)3 that demonstrates a remarkable catalyst turnover number (TON = 4,000) in the hydrogenation of MeQin under H2/CO2 conditions.
OPEN ACCESS; Selected Article
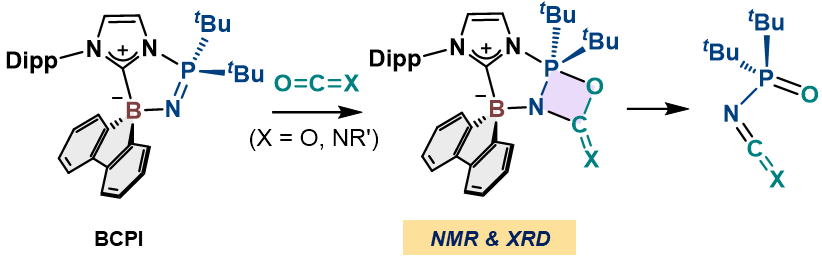
Transformation of CO2 and Isocyanates Mediated by N-Borane-Substituted Cyclic Phosphine Imides (BCPIs) via
λ5-Oxazaphosphetanes
S. Nagai, S. Ogoshi, Y. Hoshimoto
Org. Biomol. Chem. 2025, 23, 202-206.
We herein report reliable evidence
that λ5-oxazaphosphetane species are a key intermediate in the transformation
of CO2 and isocyanates through their reaction with N-borane-substituted cyclic phosphine imides
(BCPIs). Several λ5-oxazaphosphetane species were prepared via reactions
between BCPIs and CO2 or isocyanates.
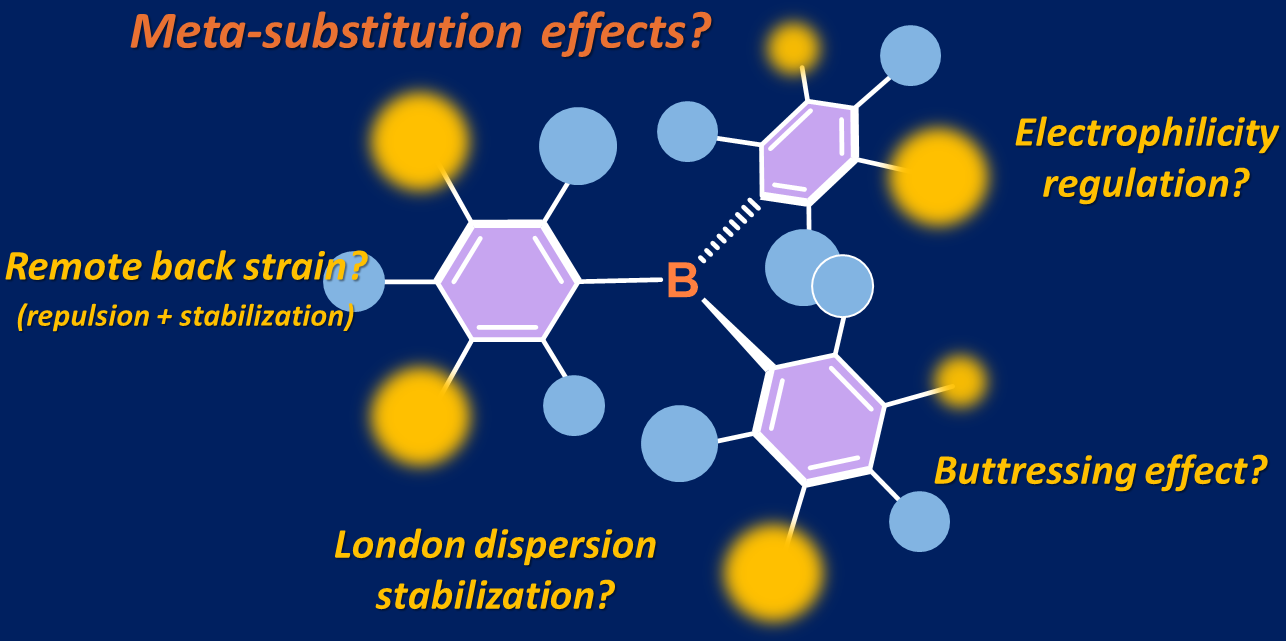
Recent Trends in Triarylborane Chemistry: Diversification of Structures and Reactivity via meta-Substitution of the Aryl Groups
M. Sakuraba, Y. Hoshimoto
Synthesis 2024, 56, 3421-3430.
This Short Review summarizes the synthesis and applications of triarylboranes
(BAr3),
including both homoleptic and heteroleptic species, with a focus on the
modification of their electronic and structural properties via the
introduction of meta-substituents with respect to the B atoms to their Ar groups.
OPEN ACCESS; Short Review; Special issue "Dual Catalysis"
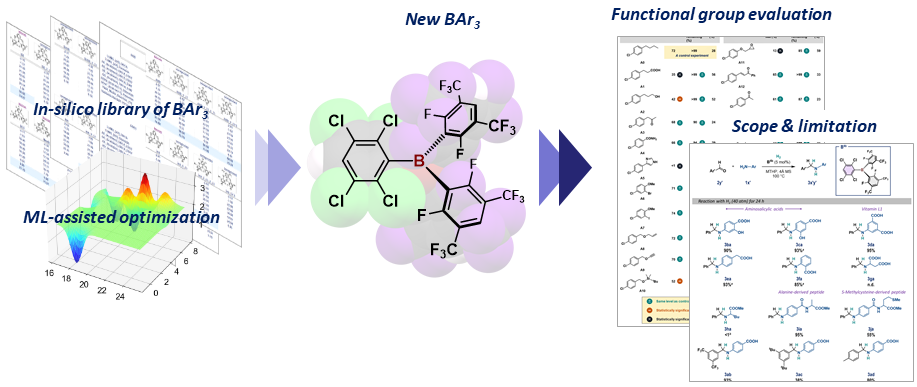
In-silico-assisted derivatization of triarylboranes for the catalytic reductive functionalization of aniline-derived amino acids and peptides with H2
Y. Hisata, T. Washio, S. Takizawa, S. Ogoshi, Y. Hoshimoto
Nature Communications, 2024,15, 3708.
Here, the construction of a triarylborane library and its application to
an ML-assisted approach for the catalytic reductive alkylation of aniline-derived
amino acids and C-terminal-protected peptides with aldehydes and H2 is reported. A combined theoretical and
experimental approach identified the optimal borane, i.e., B(2,3,5,6-Cl4-C6H)(2,6-F2-3,5-(CF3)2-C6H)2, which exhibits remarkable functional-group compatibility toward aniline
derivatives in the presence of 4-methyltetrahydropyran.
OPEN ACCESS
 concept art
concept art
Reversible Modulation of the Local Environment around Metal Centers Bearing Multifunctional Carbenes
Y. Yamauchi, S. Ogoshi, Y. Uetake, Y. Hoshimoto
Chem. Lett. 2024, 53, upae042.
This Highlight Review provides examples of
external-stimuli-driven reversible modulations of the spatial,
electronic, and magnetic environment around metal centers that bear
multifunctional N-heterocyclic carbenes (NHCs). Overall, this review highlights the multipurpose
utility of multifunctional NHCs as a key to designing and reversibly modulating
the local environment of metal centers, which paves the way to accomplishing
hitherto challenging molecular transformations and developing unprecedented
reactivity of multimetallic compounds.
OPEN ACCESS; Highlight Review; Front Cover
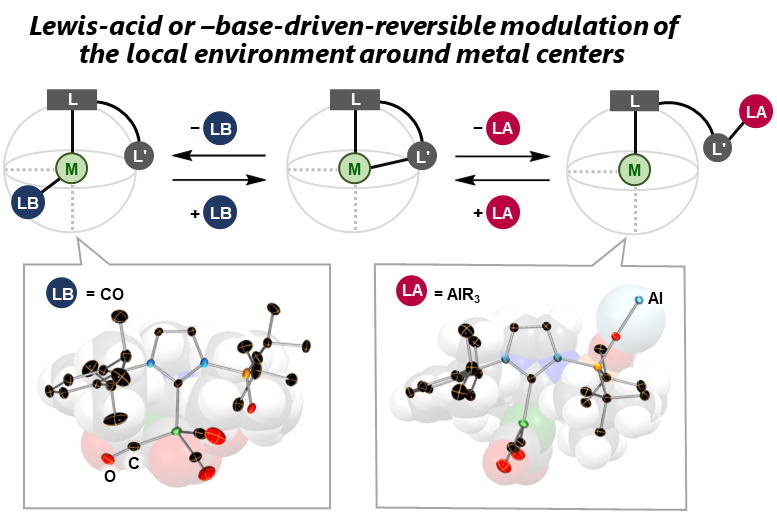

Electronic Profiling of N-Phosphine Oxide-Substituted Imidazolin-2-ylidenes (PoxIms) and Imidazolidin-2-ylidenes (SPoxIms)
J. N. Leung, Y. Mondori, S. Ogoshi, Y. Hoshimoto, H. V. Huynh
Inorg. Chem. 2024, 63, 4344.
A detailed electronic study of the N-phosphine oxide
functionalized imidazolin-2-ylidenes (PoxIms) and
imidazolidin-2-ylidenes (SPoxIms) has been performed experimentally
using IR, 13C, and 77Se NMR spectroscopies. While the net donor/acceptor properties of the (S)PoxIms
could not be differentiated via IR spectroscopy (TEP), NMR spectroscopic
methods (HEP, Se) reveal that the (S)PoxIms are slightly weaker σ-donors
but stronger π-acceptors compared to common NHCs.
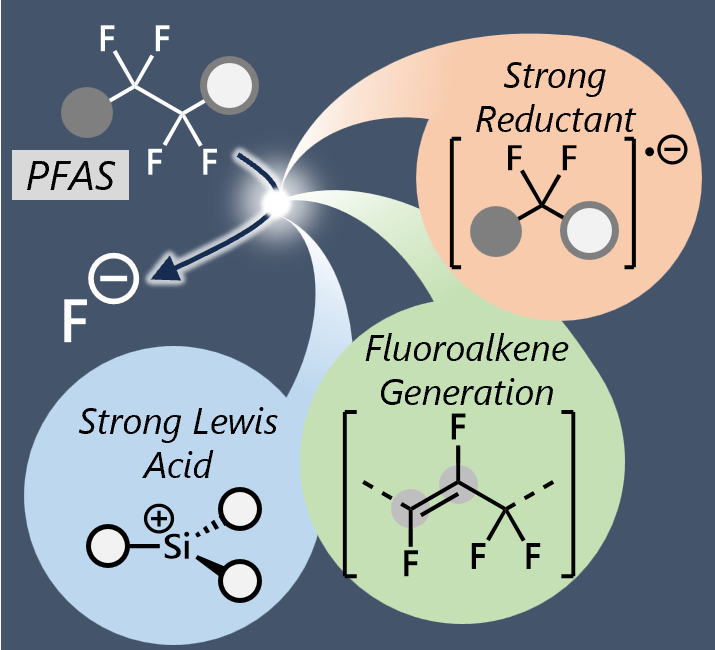
Chemical Strategies for the Cleavage of the C(sp3)−F Bonds in Perfluoroalkyl Groups
R, Doi, S. Ogoshi
Eur. J. Org. Chem., Accepted.
Perfluoroalkyl substances (PFAS) have been recognized as environmental
pollutants. Hence, their efficient and mild destruction is a significant
research interest. While many research articles and review papers have
reported the cleavage of the C(sp2)–F bonds, single C(sp3)–F bond, and
CF3 groups in PFAS, in this study, limited and emerging examples of a longer
perfluoroalkyl group containing at least one repeating unit of 1,1,2,2-tetrafluoroethylene
was focused. In this Concept, we summarized recent progress on the chemical
defluorination of PFAS via the cleavage of unactivated C(sp3)–F bonds in
longer perfluoroalkyl groups under mild conditions (~150 °C). In addition
to classical reductive defluorination, strategies featuring Lewis acid
activation and the transient generation of an unsaturated bond were described.
Palladium-Catalyzed γ-Arylation of Acylketene Synthons with Aryl Chlorides Enabled by Ylide-Functionalized Phosphines (YPhos)
S. Manna, F. Papp, Y. Hisata, J. Löffler, M. Rybka, V.H. Gessner, Y. Hoshimoto,
L. J. Gooßen
Adv. Synth. Catal. 2024, 366, 1107.
OPEN ACCESS; Very Important Publication (VIP)
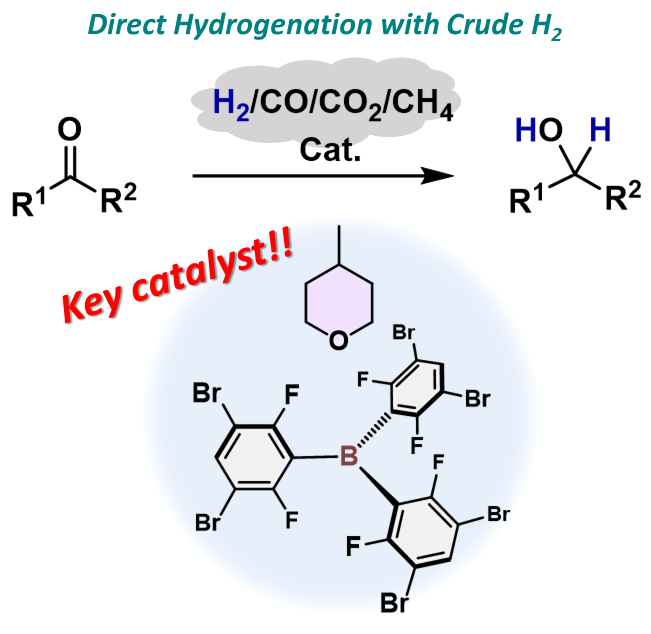
Strategic Use of Crude H2 for the Catakytic Reduction of Carbonyl Compounds
M. Sakuraba, S. Ogoshi, Y. Hoshimoto
Tetrahedron Chem, 2024, 9, 100059.
Toward a more efficient use of crude H2 without its energy-consuming purification, this study employs gaseous
mixtures of H2, CO, CO2, and CH4 for the catalytic hydrogenation of aldehydes and ketones in the presence of strategically designed triarylboranes and 4-methyltetrahydropyrane as a greener ethereal solvent. The present results emphasize the unexplored utility of less-toxic main-group catalysis for the catalytic hydrogenation using crude H2. This stands in contrast to the well-established transition-metal catalysis
that generally requires purified H2.
OPEN ACCESS; Invited contribution to "Organocatalysis"
N-Heterocyclic Carbenes with Polyfluorinated Groups at the 4- and 5-Positions from [3 + 2] Cycloadditions between Formamidinates and cis-1,2-Difluoroalkene Derivatives
M. Ohashi, K. Ando, S. Murakami, K. Michigami, S. Ogoshi
J. Am. Chem. Soc. 2023, 145, 23098-23108.
We herein report the formation of fluorinated N-heterocyclic carbenes (NHCFs)
that bear fluorine atoms at the 4- and 5-positions of the imidazol-2-ylidene
ring. The fluorine substituents, contrary to expectations, tend to act
as electron donors owing to the considerable positive mesomeric effect,
while the perfluorocyclopentene-fused and tetrafluorobenzo-fused rings
are pure electron acceptors due to their strong negative inductive effect.
Moreover, an analysis of the % buried volume (%Vbur) values clearly suggests
that the modification of the NHC backbone with polyfluorinated groups can
drastically alter the electronic properties of the NHC ligand without substantially
changing its steric properties. Our experimental results were further corroborated
by a series of computational calculations.
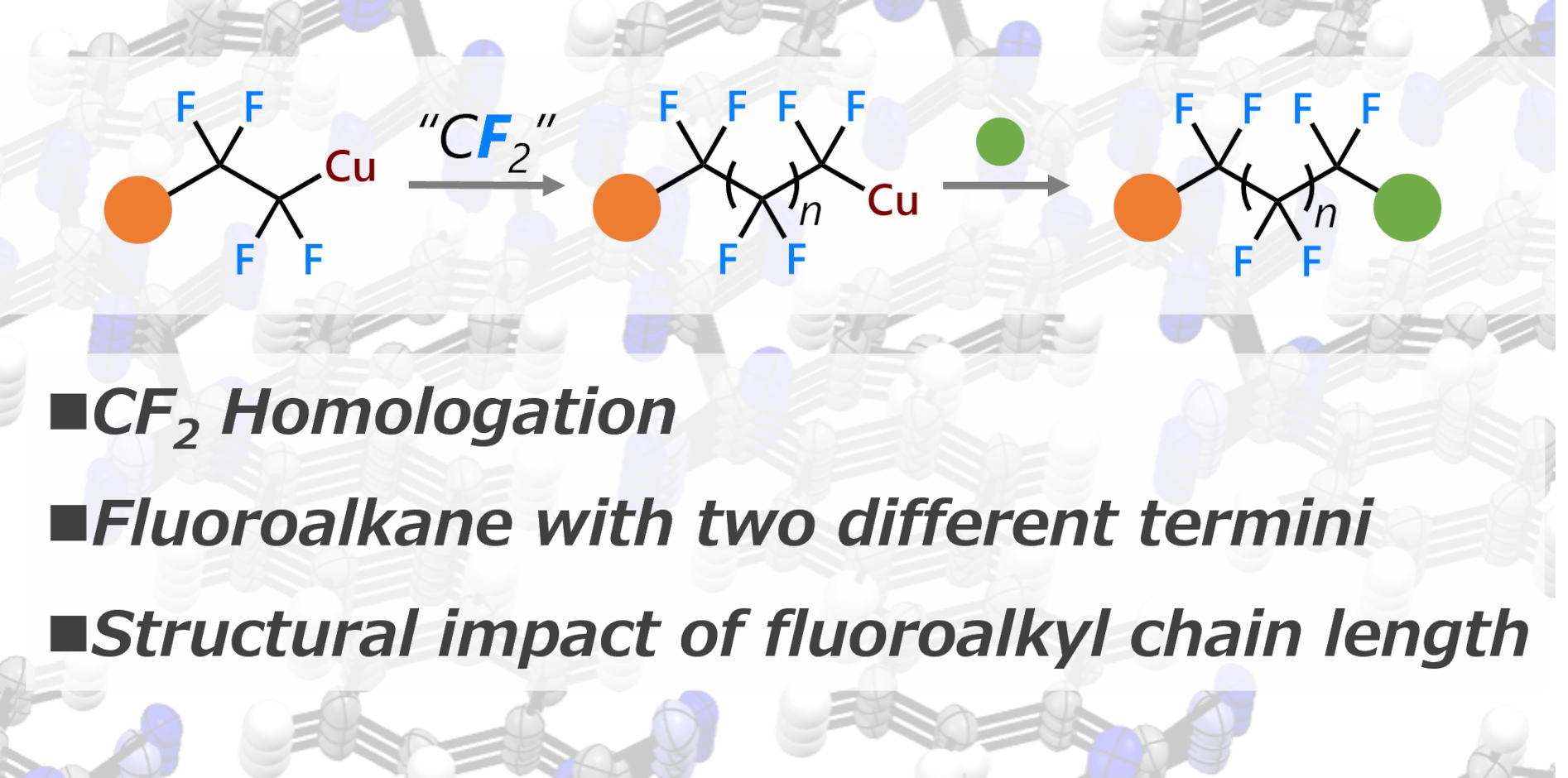
Difluoromethylene Insertion into Fluoroalkyl Copper Complexes
Y. Zhou, R. Doi, S. Ogoshi
Chem. Commun.2023, 59, 11504. (DOI: 10.1039/D3CC03481J).
Herein, we report the insertion of a difluoromethylene into 1,1,2,2-tetrafluoro-2-arylethyl
copper complexes to synthesize extended perfluoroalkyl-bridged compounds
that have various functional groups on each edge. Further, the one-pot
syntheses of perfluoroalkyl-bridged compounds from aryl boronic acid esters
were carried out.
Selected as "Chemical Communications HOT Articles 2023"
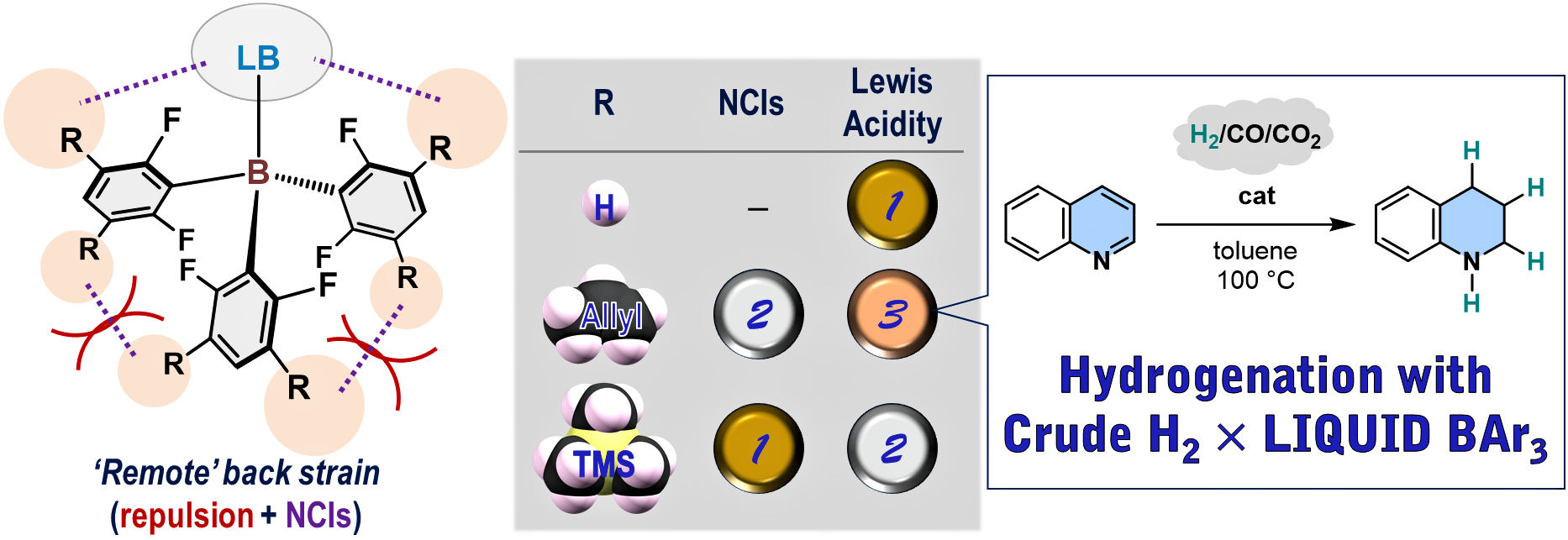
Remote Back Strain: A Strategy for Modulating the Reactivity of Triarylboranes
M. Sakuraba, T. Morishita, T. Hashimoto, S. Ogoshi, Y. Hoshimoto
Synlett 2023, 34, 2187-2192.
A strategy for modulating the Lewis acidity of triarylboranes is proposed
based on the concept of ‘remote’ back strain. Applying this concept, we
synthesized B(2,6-F2-3,5-TMS2-C6H)3 and the liquid B(2,6-F2-3,5-allyl2-C6H)3
and demonstrated their superior catalytic activity for the hydrogenation
of quinoline relative to B(C6F5)3 and B(2,6-F2-C6H3)3.
Invited contribution to "Modern Boron Chemistry: 60 Years of the Matteson
Reaction"
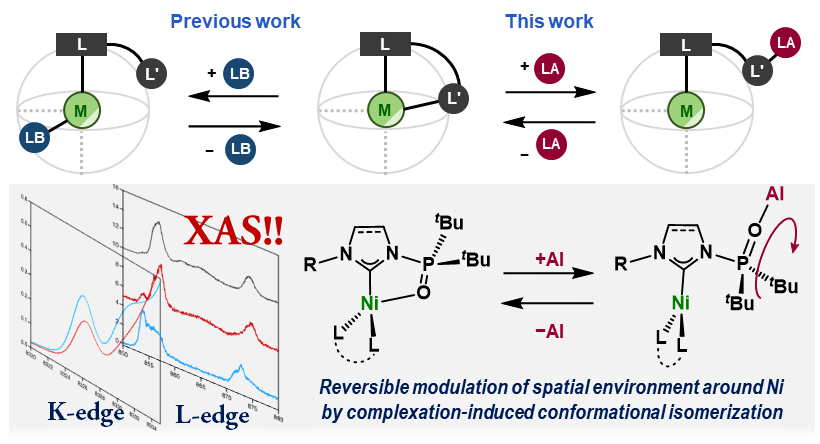
Reversible Modulation of the Electronic and Spatial Environment around Ni(0) Centers Bearing Multifuctional Carbene Ligands with Triarylaluminum
Y. Yamauchi, Y. Mondori, Y. Uetake, Y. Takeuchi, T. Kawakita, H. Sakurai,
S. Ogoshi, Y. Hoshimoto
J. Am. Chem. Soc. 2023, 143, 16938.
ChemRxiv. 2023, preprint (DOI: 10.26434/chemrxiv-2023-5nz52).
We demonstrate a Lewis-acid-mediated reversible expansion, contraction,
and transformation of the spatial environment surrounding nickel(0) centers
that bear N-phosphine oxide-substituted N-heterocyclic carbenes. The multinuclear NMR, IR, and XAS analyses clarified the details of the
changes in the electronic states on the Ni centers. The results presented
in this work thus provide a strategy for reversibly modulating the electronic
and spatial environment of organometallic complexes, in addition to the
well-accepted Lewis-base-mediated ligand-substitution methods.
OPEN ACCESS
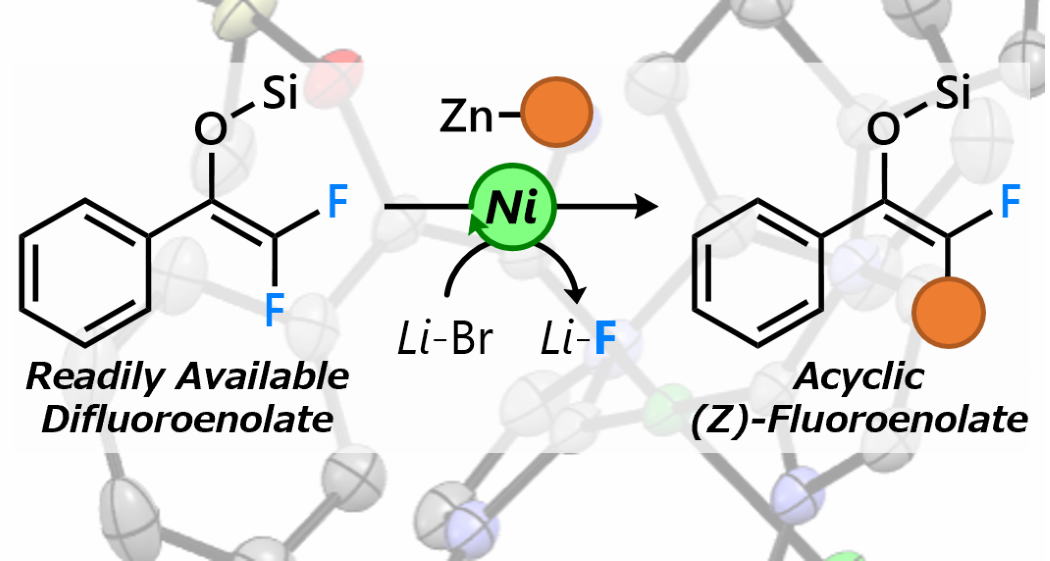
Regioselective C-F Bond Transformations of Silyl Difluoroenolates
R. Doi, K. Kajiwara, T. Negoro, K. Koh, S. Ogoshi
Org. Lett. 2023, 25, 5542.
ChemRxiv. 2023, preprint (DOI: 10.26434/chemrxiv-2023-tszsc).
Herein, we report the development of a nickel-catalyzed cross-coupling
reaction of silyl difluoroenolates with aryl zinc reagents via C−F bond
cleavage. Treatment of a stoichiometric amount of Ni(0)/ N-heterocyclic
carbene (NHC) with silyl difluoroenolates in the presence of a lithium
salt resulted in C−F bond cleavage to selectively afford the corresponding
(Z)-alkenyl Ni complexes. Density functional theory (DFT) calculations
revealed the importance of the silyl substituent in creating environments
with varying steric hindrance for each of the two fluorine atoms. Based
on these observations, we developed a catalytic cross-coupling reaction
that selectively delivers a single geometric isomer of a fluoroenolate.
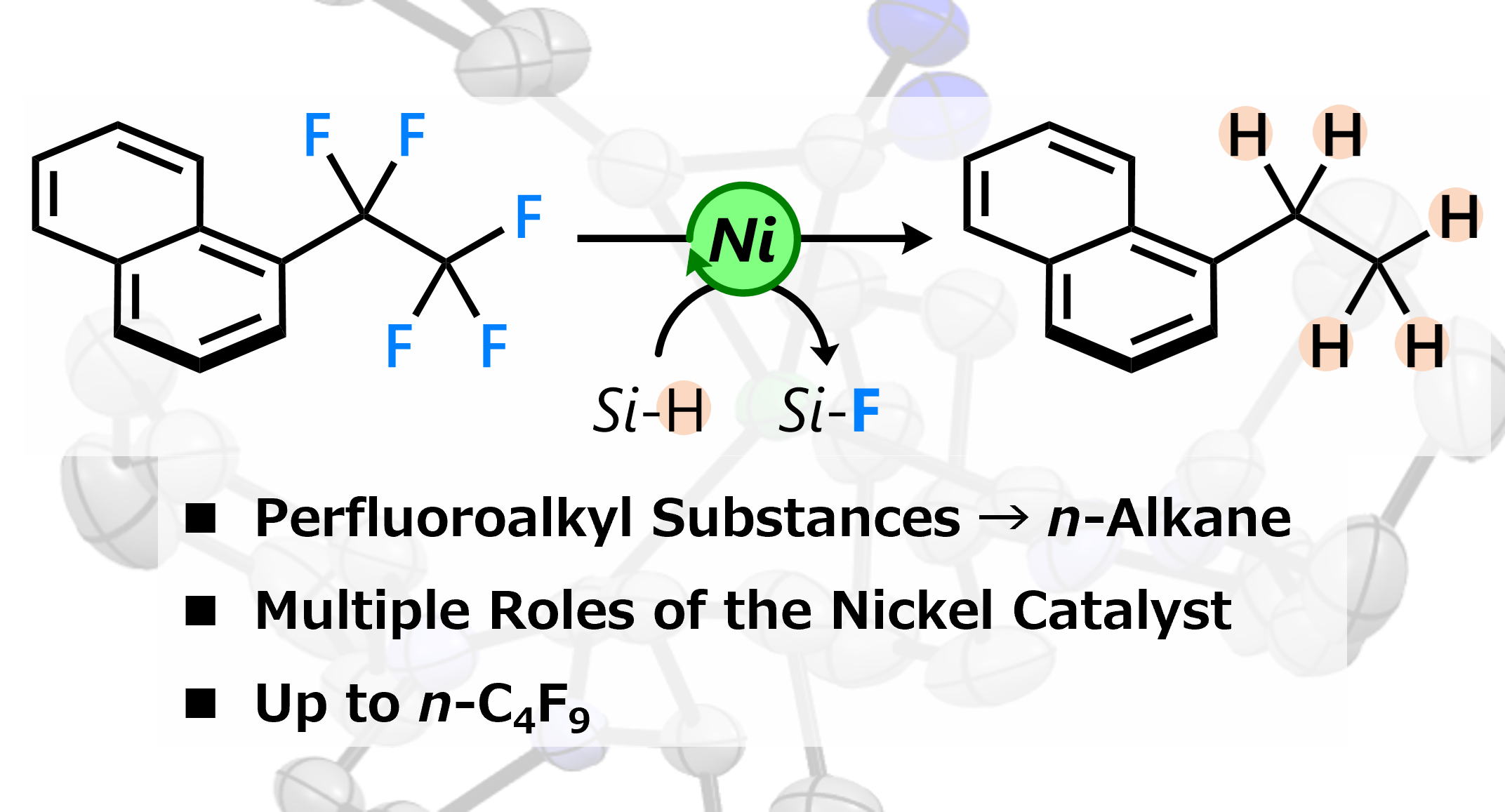
Nickel-catalyzed exhaustive hydrodefluorination of perfluoroalkyl arenes
R. Doi, M. Yasuda, N. Kajita, K. Koh, S. Ogoshi
J. Am. Chem. Soc. 2023, 145, 11449-11456.
ChemRxiv. 2023, preprint (DOI: 10.26434/chemrxiv-2023-9tkg4).
Perfluoroalkyl compounds are persistent environmental pollutants due to
their strong C(sp3)−F bonds. Hydrodefluorination has emerged as a potential
alternative disposal method for perfluoroalkyl compounds. Although the
transformation of trifluoromethyl arenes into the corresponding methyl
arenes has been studied by several research groups, hydrodefluorination
reactions of longer perfluoroalkyl chains remain rare. Herein, we report
exhaustive hydrodefluorination reactions of pentafluoroethyl arenes and
longer-chain analogues using molecular nickel catalysis. Despite the cleavage
of multiple C(sp3)−F bonds, the reaction already proceeds upon gentle heating.
A mechanistic investigation indicated that the reaction proceeds via benzylic
hydrodefluorination reactions followed by homobenzylic ones. We reveal
the multiple roles of the Ni catalyst, which include C−F bond cleavage,
promotion of HF elimination, and hydrosilylation.
Photocatalyst-Free Visible Light-Mediated C-H Perfluoroalkylation of Quinazolin-4(3H)-ones with perfluoroalkyl iodides
T. Delouche, A. Gadiry-Diallo, T. Besson, S. Ogoshi, C. Fruit
Synthesis 2023, 55, 3670-3684.
A practical and sustainable photocatalyst-free protocol for photo-induced synthesis of perfluoroalkylated quinazolin-4(3H)-ones is described starting from quinazolin-4(3H)-ones. A wide range of substituted or fused-quinazolinones is found to be compatible, providing the corresponding mono- and bis-perfluoroalkylated compounds in moderate yields. This visible-light mediated C-H perfluoroalkylation allows an environmentally friendly and straightforward access to an array of unprecedented functionalized quinazolinone scaffolds, presenting attractive features for drug discovery. The control experiments demonstrated that a radical mechanism is involved in the reaction mechanism.
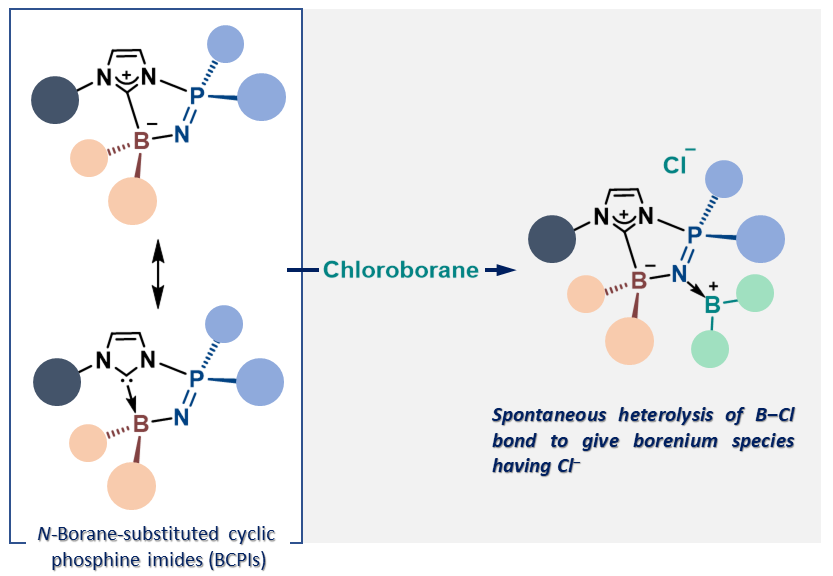
N-Borane-Substituted Cyclic Phosphine Imides (BCPIs)
S. Nagai, T. Hinogami, S. Ogoshi, Y. Hoshimoto
Bull. Chem. Soc. Jpn. 2023, 96, 1346-1353.
ChemRxiv. 2023, preprint (DOI: 10.26434/chemrxiv-2023-qv422-v2).
Herein, we have designed and prepared a novel class of phosphine imides
known as N-borane-substituted cyclic phosphine imides (BCPIs). Given a
characteristic nucleophilic/Lewis basic reactivity of BCPIs, although it
has been previously considered unlikely, the spontaneous heterolysis of
a B‒Cl bond in a BCPI-coordinated chloroborane has been directly observed,
suggesting that such process is a plausible key step in the Lewis acid-promoted
generation of borenium species from chloroboranes.
OPEN ACCESS; Selected Paper; Inside Cover
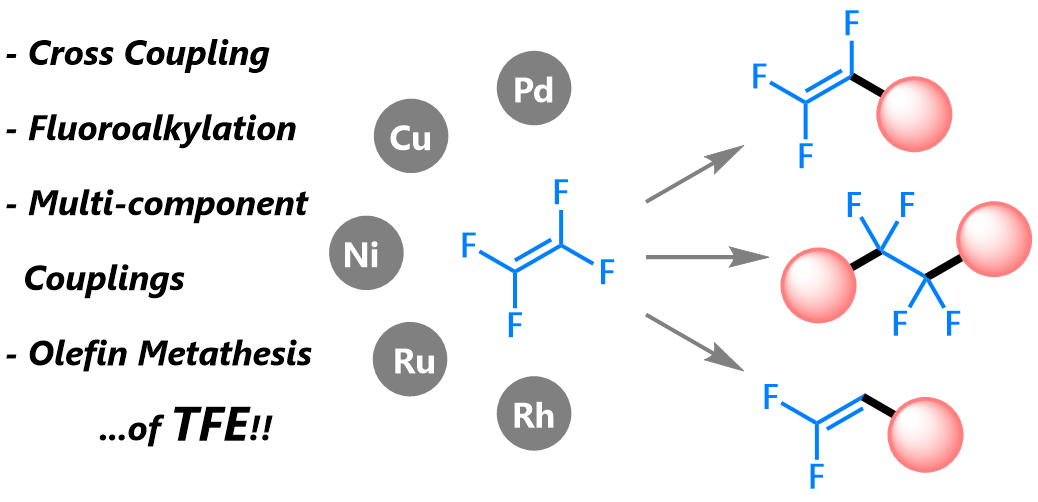
Transformation of Tetrafluoroethylene using Transition-Metal Complexes
R. Doi, Y. Zhou, S. Ogoshi
Synthesis 2023, 55, 857.
This review highlights studies on the transformation of TFE into organofluorine
compounds using transition-metal complexes, except for polymerizations.
Our review covers cross-coupling reactions via C−F bond cleavage, fluoroalkylation
reactions, multi-component couplings, and olefin metathesis.
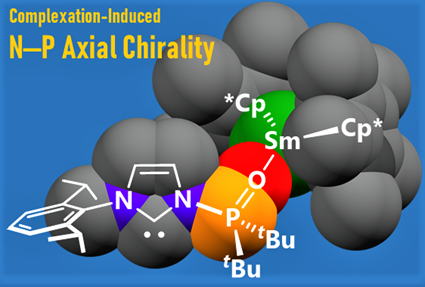
Complexation-Induced N-P Axial Chiraity in Sm(II) N-Phosphine-Oxide-Substituted Imidazolylidene and Imidazolinylidene Complexes
Y. Hoshimoto, Y. Yamauchi, T. Terada, S. Ogoshi
Can. J. Chem. 2022, 101, 429-433.
ChemRxiv. 2022, preprint (DOI: 10.26434/chemrxiv-2022-rf1wd)
Two types of Sm(II) complexes bearing either an N-phosphine-oxide-substituted imidazolylidene (PoxIm) or the corresponding
imidazolinylidene (SPoxIm) were successfully prepared and characterized
using single crystal X-ray diffraction analysis. In some cases, axial chirality
was clearly induced around the N-P bonds.
Invited contribution to "the Crudden Special Issue"
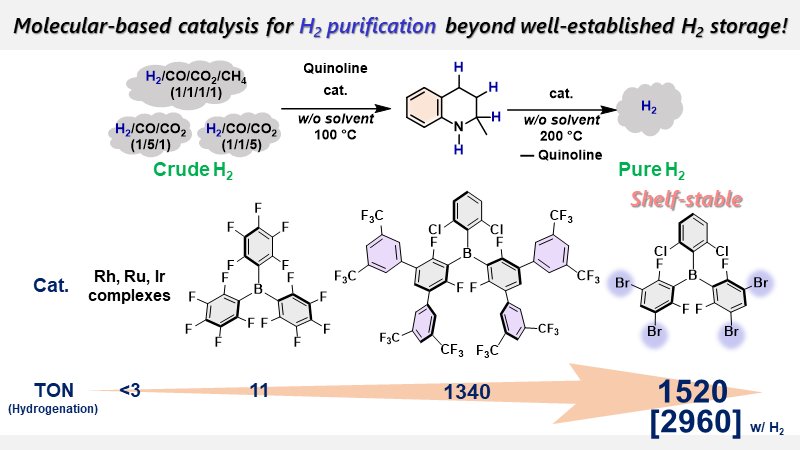
Main-group catalysis for H2 purification based on liquid organic hydrogen carriers
T. Hashimoto, T. Asada, S. Ogoshi, Y. Hoshimoto
Sci. Adv. 2022, 8, eade0189.
ChemRxiv. 2022, preprint (10.26434/chemrxiv-2022-pgbgd)
Here, we demonstrate a strategy to separate H2 from a gaseous mixture of H2/CO/CO2/CH4 and
simultaneously store it in N-heterocyclic
compounds that act as liquid organic hydrogen carriers (LOHCs), which can be
applied to produce H2 by subsequent dehydrogenation. A newly
designed shelf-stable triaryl borane is able to catalyze the hydrogenation of
the N-heteroaromatic compounds in the
co-presence of substantial amounts of CO, CO2, CH4, and H2O
(i.e., the simultaneous separation and storage of H2), and to promote the subsequent dehydrogenation (i.e., H2 recovery), both of which proceed under the solvent-free conditions.
OPEN ACCESS; Press Release;Featured at EurekAlert; AlphaGalileo; Asia Research News; PhysOrg; Scienmag; Nanowerk; 時事通信; YAHOO!; Cosmos Magazine; 日経xTech; Chem-Station;現代化学(東京化学同人, 2023年1月号)

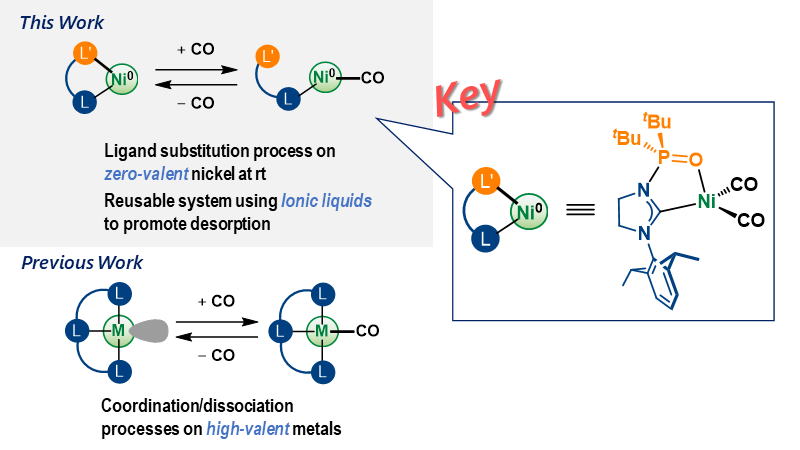
Room-Temperature Reversible Chemisorption of Carbon Monoxide on Nickel(0) Complexes
Y. Yamauchi, Y. Hoshimoto, T. Kawakita, T. Kinoshita, Y. Uetake, H. Sakurai,
S. Ogoshi
J. Am. Chem. Soc. 2022, 144, 8818-8826.
ChemRxiv. 2022, preprint (10.26434/chemrxiv-2022-nb0fw)
Herein, we report a pressure-responsive nickel(0)-based system that is
able to reversibly chemisorb carbon monoxide (CO) at room temperature.
The use of N-heterocyclic carbene ligands with hemi-labile N-phosphine
oxide substituents facilitates both the adsorption and desorption of CO
on nickel(0) via ligand substitution. Ionic liquids were used as the
reaction medium to enhance the desorption rate and establish a reusable
system.
OPEN ACCESS; Press Release;現代化学(東京化学同人, 2022年7月号);Chem-Station
Overlooked Factors Required for Electrolyte Solvents in Li-O2 Battery: Capabilities of Quenching 1O2 and Forming Highly-decomposable Li2O2
K. Nishioka, M. Tanaka, H. Fujimoto, T. Amaya, S. Ogoshi, M. Tobisu, S.
Nakanishi
Angew. Chem. Int. Ed. 2022, 61, e202112769..
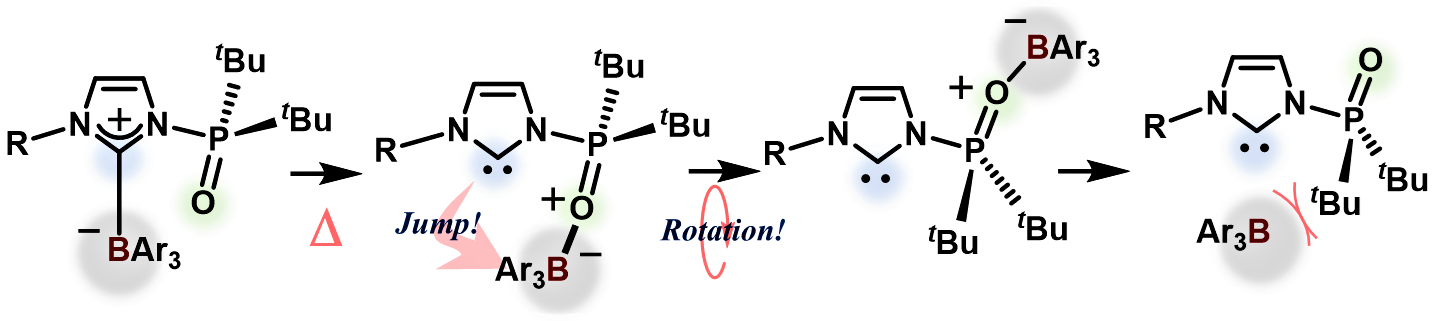
A Boron-Transfer Mechanism Mediating the Thermally Induced Revival of Frustrated Carbene−Borane Pairs from their Shelf-Stable Adducts
Y. Hoshimoto, M. Sakuraba, T. Kinoshita, M. Ohbo, M. Ratanasak, J. Hasegawa,
S. Ogoshi
Commun. Chem.2021, 4, 137.
ChemRxiv. 2021, preprint (DOI: 10.26434/chemrxiv.14462391.v1)
Combined experimental and theoretical studies allowed clarifying the reaction
mechanism for the revival of frustrated carbene−borane pairs from external-stimuli-responsive
classical Lewis adducts comprised of N-phosphine oxide-substituted imidazolylidenes
and triarylboranes. A borane-transfer process from the carbene carbon atom
to the N-phosphinoyl oxygen atom was identified as the rate-determining
event for the regeneration of the FLP species, eventually enabling the
heterolytic cleavage of H2.
Open Access
多用途な多官能基化環状カルベンPoxImの開発
山内 泰宏、星本 陽一、生越 專介
有機合成化学協会誌, 2021, 79, 632-641. (in Japanese; 総合論文)
Practical methods have been established for the synthesis of multifunctional N-heterocyclic
carbenes (NHCs) via the introduction of substituents on either the
nitrogen atom(s) or the backbone of the NHCs. However, their use has
been mainly limited to acting as multidentate ligands for metal
complexes. Herein, results of our recent studies on the synthesis and
application of N-phosphine oxide-substituted imidazolylidenes (PoxIms) as novel type of
multifunctional NHCs are discussed.
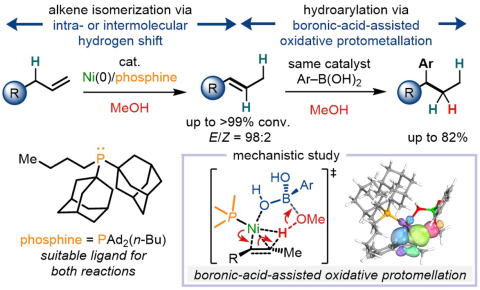
Development and Mechanistic Studies of E-Selective Isomerization/Tandem Hydroarylation Reactions of Alkenes with a Nickel(0)/Phosphine Catalyst
H. Iwamoto, T. Tsuruta, S. Ogoshi
ACS Catal. 2021, 11, 6741-6749.
Herein, we report the reaction of terminal alkenes with the nickel(0)/PAd2(n-Bu) catalyst selectively afforded the corresponding (E)-alkenes. Furthermore, sequential hydroarylations occured when aryl boronic acids were added under the same reaction conditions. A combined experimental and computational mechanistic studies suggested that the isomerization reactions occurred via an intramolecular 1,3-hydrogen shift and an intermolecular hydrogen shift. Moreover, a concerted multi-bond recombination with boronic-acid-assisted oxidative protometallation of the alkene was newly found as an important step for the formation of the alkylnickel(II) species in the hydroarylation.
Sm(II)-Mediated Single-Electron Reduction of Pentafluorophenylcopper(I)
Y. Yamauchi,† S. Nagai† T. Terada, Y. Hoshimoto, S. Ogoshi
(†These authors equally contributed to this work)
Chem. Lett. 2021, 50, 1394-1396.
The single-electron-transfer reduction of pentafluorophenylcopper(I) using
the Sm(II) reagents resuted in the formation of ion-paired [Sm(II)][Cu(C6F5)2] species with the concomitant generation of Cu(0).
Open Access
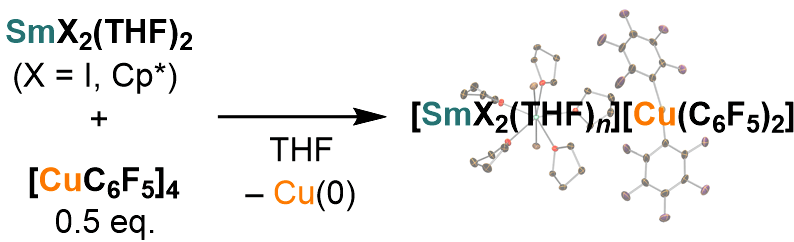
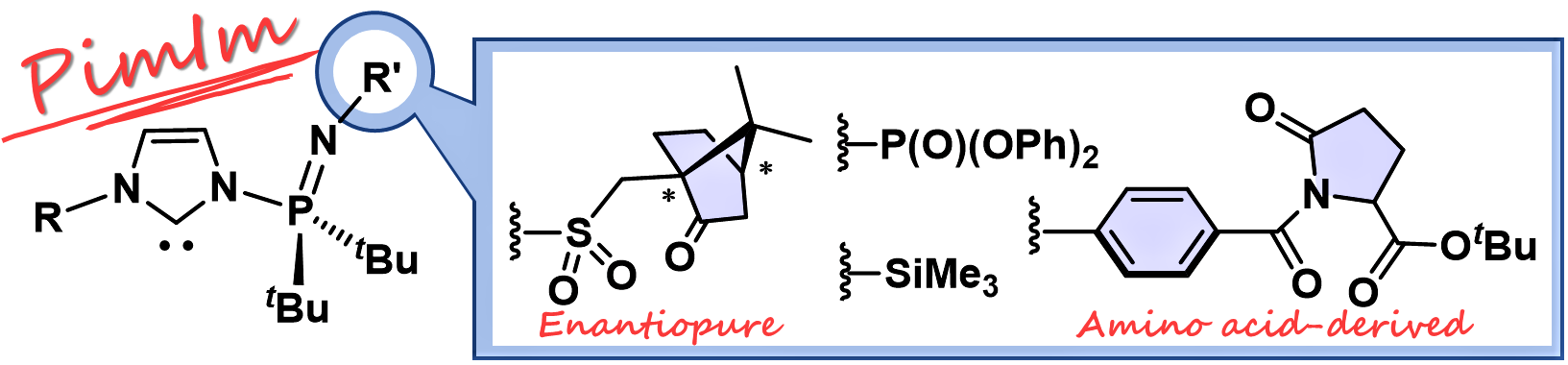
N‐Phosphine Imide‐Substituted Imidazolylidenes (PimIms)
Y. Hoshimoto, S. Nagai, T. Hinogami, S. Hazra, S. Ogoshi
Asian J. Org. Chem. 2021, 10, 1085-1089.
A series of novel multifunctional carbenes of the type N-phosphine imide‐substituted imidazolylidenes (PimIms) has been synthesized
and characterized. Substituents including (+)-10-camphorsulfonyl and pyroglutamate
groups were introduced on the N-phosphinimidoyl moiety to diversify available structures of isolable carbenes.
Invited contribution to "Early Carrer Special Collections"
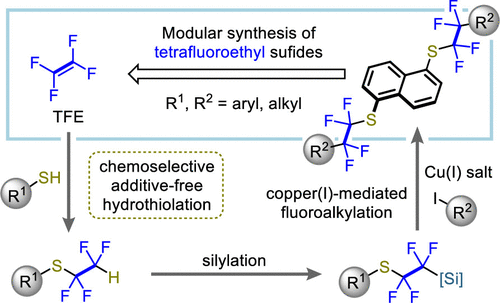
Synthesis of Fluoroalkyl Sulfides via Additive-Free Hydrothiolation and Sequential Functionalization Reactions
D. E. Sunagawa, N. Ishida, H. Iwamoto, M. Ohashi, C. Fruit, S. Ogoshi
J. Org. Chem. 2021, 86, 6015-6024.
A modular synthetic method, involving a hydrothiolation, silylation, and
fluoroalkylation, for the construction of highly functionalized fluoroalkyl
sulfides has been developed. The use of aprotic polar solvents enables
the additive-free chemoselective hydrothiolation of tetrafluoroethylene,
trifluorochloroethylene, and hexafluoropropene with various thiols. The
stepwise functionalization reactions convert the hydrothiolated intermediates
into the tetrafluoroethyl sulfides in high efficiency. The method avoids
the use of the environmental pollutant Halon-2402, which was employed as
a building block in a reported synthetic route.
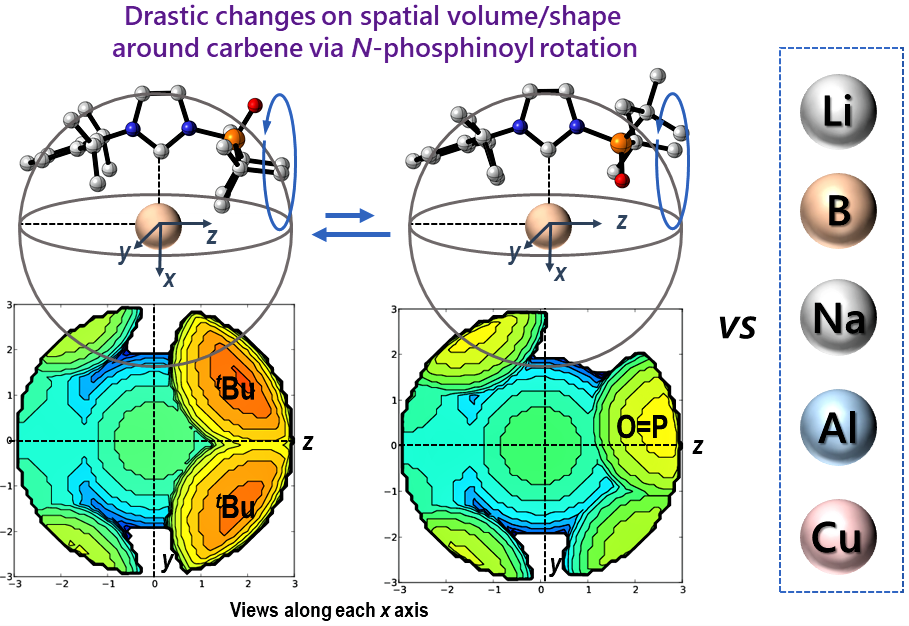
Development of Metal Complexes Equipped with Structurally Flexible Carbenes
Y. Hoshimoto, S. Ogoshi
Bull. Chem. Soc. Jpn. 2021, 94, 327-338
We herein summarize our recent results on the design and application of metal complexes that bear N-phosphine-oxide-substituted
imidazolylidenes (PoxIms), in which the volume and shape of the
reaction space around the carbene atoms can be drastically changed via
the rotation of the N-phosphinoyl groups; this phenomenon is discussed in detail based on experimental
and theoretical results.
Open Access; Award Accounts; Inside Cover
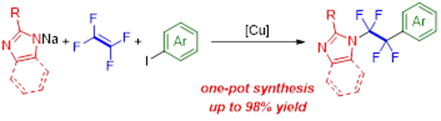
Copper(I)-Mediated C–N/C–C Bond-Forming Reaction with Tetrafluoroethylene for the Synthesis of N-Fluoroalkyl Heteroarenes via an Azacupration/Coupling Mechanism
N. Ishida, T. Adachi, H. Iwamoto, M. Ohashi, S. Ogoshi
Chem. Lett. 2021, 50, 442-444.
A novel copper-mediated N-fluoroalkylation
of heteroarenes has been developed. Treating an in-situ-generated N–Cu
complex with tetrafluoroethylene (TFE) in the presence of iodoarenes furnished
the corresponding N- and aryl-difunctionalized tetrafluoroethanes in up to 98% yield. This
method enables the synthesis of tetrafluoroethylene-bridged heteroarenes
in a one-pot reaction.