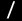Publication
2021-2025 |2016-2020 | 2015-2011 | 2010-2006 | 2005-2003
Nickel(0)/N-Heterocyclic Carbene-Catalyzed Asymmetric [2+2+2] Cycloaddition of Two Enones and an Alkyne: Access to Cyclohexenes with Four Contiguous Stereogenic Centers
R. Kumar, H. Tokura, A. Nishimura, T. Mori, Y. Hoshimotoi, M. Ohashi, and
S. Ogoshi
Org. Lett. 2015, 17, 6018.
A catalytic enantioselective [2+2+2] cycloaddition of two enones and an
alkyne has been devised to access enantioenriched cyclohexenes with the
generation of four contiguous stereogenic centers. The reaction proceeds
with the oxidative cyclization of an enone and an alkyne with a chiral nickel/NHC
complex followed by insertion of another enone.
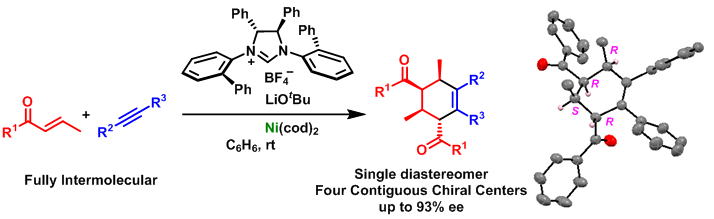
Nickel(0)-Catalyzed Enantio- and Diastereoselective Synthesis of Benzoxasiloles: Ligand-Controlled Switching from Inter- to Intramolecular Aryl-Transfer Process
R. Kumar, Y. Hoshimoto, H. J. Yabuki, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2015, 137, 11838-11845.
A highly enantioselective synthesis of 3-aryl-, vinyl- and alkynyl-2,1-benzoxasiloles
was achieved via the sequential activation of an aldehyde and a silane
by nickel(0). Initial mechanistic studies revealed the complete switching
of an aryl-transfer process from an intermolecular (racemic synthesis:
JACS 2014) to an intramolecular (enantioselective synthesis) fashion.
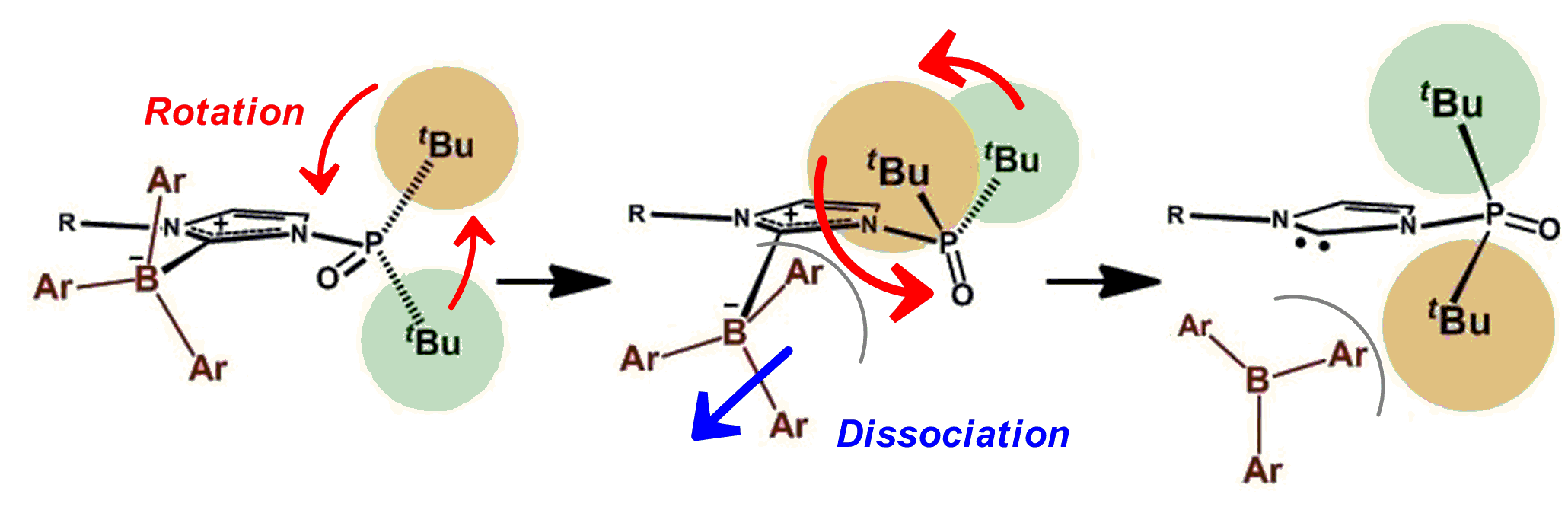
A Strategy to Control the Reactivationl of Frustrated Lewis Pairs from Shelf-Stable Carbene-Borane Complexes
Y. Hoshimoto, T. Kinoshita, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2015, 54, 11666-11671.
OPEN ACCESS
A strategy to reactivate frustrated Lewis pairs from classical Lewis adducts
by exploiting molecular motions that are responsive to external stimuli
has been developed with N-phosphine oxide substituted imidazolylidene (PoxIm).
The reactivation conditions were successfully controlled by tuning the
strain in the PoxIm–B(C6F5)3 complexes.
Highlighted as a Front Cover Picture
Highlighted on Altas of Science: "Control the frustration between
molecular pairs with external stimuli-responsive motions"
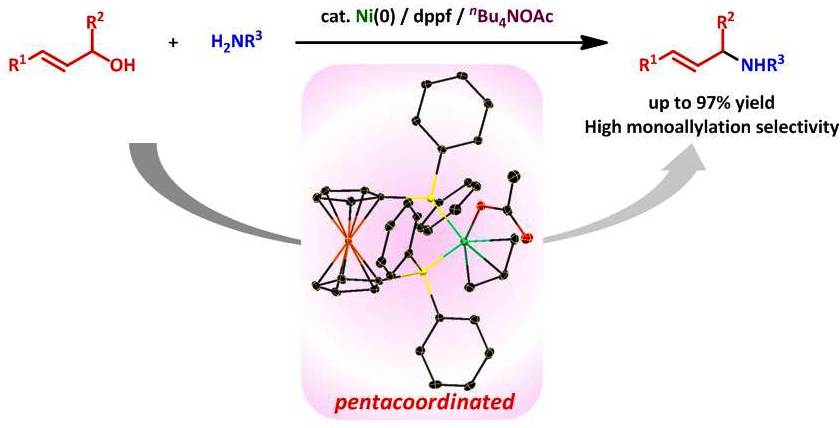
Pentacoordinated Carboxylate π-allyl-Allyl Nickel Complexes as Key Intermediates for Ni-catalyzed Direct Amination of Allylic Alcohols
Y. Kita, H. Sakaguchi, Y. Hoshimoto, D. Nakauchi, Y. Nakahara, J.-F. Carpentier,
S. Ogoshi, K. Mashima
Chem. Eur. J. 2015, 21, 14571-14578.
Direct amination of allylic alcohols with primary and secondary amines
catalyzed by a system made of Ni(cod)2 and DPPF was effectively enhanced
by adding nBu4NOAc and molecular sieves, affording the corresponding allylamines.
Such remarkable additive effects of nBu4NOAc were elucidated by isolating
and characterizing some nickel complexes, manifesting the key role of a
charge neutral pentacoordinated η3-allyl acetate complex in the present system.
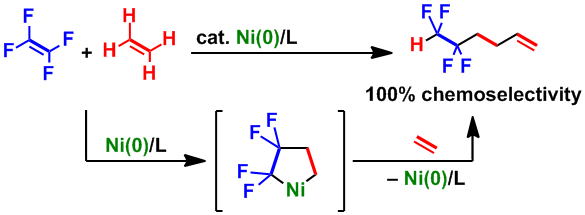
Nickel-Catalyzed Formation of Fluorine-Containing Ketones via the Selective Cross-Trimerization Reaction of Tetrafluoroethylene, Ethylene, and Aldehydes
M. Ohashi, H. Shirataki, K. Kikushima, and S. Ogoshi
J. Am. Chem. Soc. 2015, 137, 6496-6499.
In the presence of a catalytic amount of Ni(cod)2 and IPr, a cross-trimerization reaction of tetrafluoroethylene, ethylene, and aldehydes
proceeded in a selective manner to afford a variety of 4,4,5,5-tetrafluoro-1-pentanone derivatives in good to excellent yields. This system involves a five-membered
nickelacycle key intermediate generated via the oxidative cyclization of tetrafluoroethylene and ethylene.
Highlight: C&E NEWS 2015, 93, 28. "Tetrafluoroethylene Is Good For More Than Just Teflon"

Catalytic Transformation of Aldehydes with Nickel Complexes through η2 Coordination and Oxidative Cyclization
Y. Hoshimoto, M. Ohashi, and S. Ogoshi
Acc. Chem. Res. 2015, 48, 1746-1755.
Special Issue"Earth Abundant Metals in Homogeneous Catalysis"
2,2,3,3-Tetrafluoronickelacyclopentanes Generated via the Oxidative Cyclization of Tetrafluoroethylene and Simple Alkenes: A Key Intermediate in Nickel-Catalyzed C-C Bond-Forming Reactions
M. Ohashi, T. Kawashima, T. Taniguchi, K. Kikushima, and S. Ogoshi
Organometallics 2015, 34, 1604-1607.
In the presence of PPh3, the partially fluorinated five-membered nickelacycle generated via the oxidative cyclization of tetrafluoroethylene (TFE) and ethylene
with Ni(0) was isolated and the structure was determined by X-ray analysis. This nickelacycle was found not only to react stoichiometrically with enones to give a cross-trimerization product,
but also to be a key reaction intermediate in the Ni(0)-catalyzed co-trimerization of TFE and ethylene, leading to 5,5,6,6-tetrafluoro-1-hexene.
Highlight: C&E NEWS 2015, 93, 28. "Tetrafluoroethylene Is Good For More Than Just Teflon"
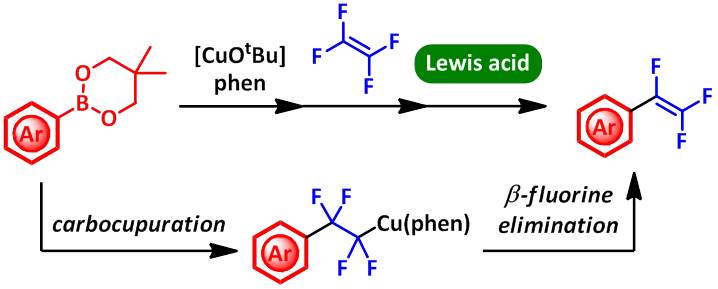
Copper-Mediated One-Pot Synthesis of Trifluorostyrene Derivatives from Tetrafluoroethlene and Arylboronate
K. Kikushima, H. Sakaguchi, H. Saijo, M. Ohashi, and S. Ogoshi
Chem. Lett. 2015, 44, 1019-1021.
The copper-mediated synthesis of trifluorostyrene derivatives was developed.
β-Fluorine elimination of 2-aryl-1,1,2,2-trifluoroethylcopper complex,
generated in situ from arylboronate, CuOtBu, and 1,10-phenanthroline with tetrafluoroethylene via carbocupration, was promoted by the addition of Lewis acid.
The present reaction system was applied to the one-pot synthesis of various trifluorostyrene derivatives, through the transmetalation–carbocupration–β-fluorine elimination sequence.
Aza-Nickelacycle Key Intermediate in Nickel(0)-Catalyzed Transformation Reactions
M. Ohashi, Y. Hoshimoto and S. Ogoshi
Dalton Trans. 2015, 44, 12060-12073.
Synthesis, Characterization, and Unique Catalytic Activities of a Fluorinated Nickel Enolate
R. Doi, K. Kikushima, M. Ohashi and S. Ogoshi
J. Am. Chem. Soc. 2015, 137, 3276-3282.
We have synthesized a new nickel enolate [(PhCOCF2)Ni(dcpe)][FB(C6F5)3] featuring
fluorine atoms on the enolate moiety via B(C6F5)3-promoted C-F bond activation of
α,α,α-trifluoroacetophenone.
We established unique catalytic applications for [(PhCOCF2)Ni(dcpe)][FB(C6F5)3]
toward a Tishchenko reaction, along with a highly-selective crossed-esterification of α,α,α-trifluoroacetophenones with aldehydes.
Catalytic Transformations of Fluorinated Olefins
M. Ohashi and S. Ogoshi
Topics in Organomet. Chem. 2015, 52, 197-216
Progress in the development of catalytic transformation reactions of fluorinated olefins is reviewed.
Four different types of transition-metal-catalyzed transformations have been distinguished:
(a) oligomerization, (b) hydrodefluorination, (c) C-X bond formation via C-F bond cleavage,
and (d) C-C bond formation via C-F bond cleavage.
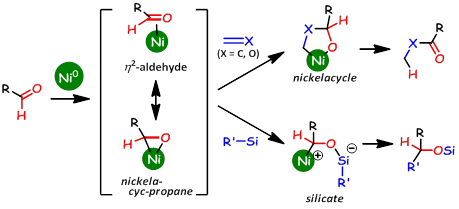
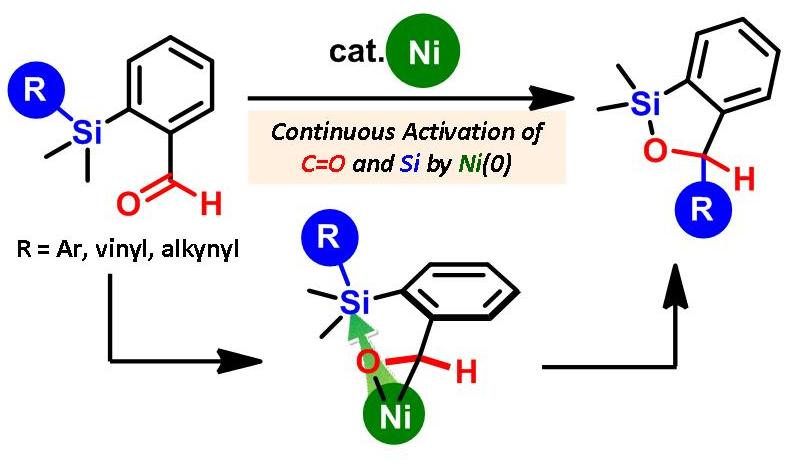
Highly Efficient Activation of Organosilanes with η2-Aldehyde Nickel Complexes: Key for Catalytic Syntheses of Aryl-, Vinyl-, and Alkynyl-Benzoxasiloles
Y. Hoshimoto, H. J. Yabuki, R. Kumar, H. Suzuki, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2014, 136, 16752.
An η2-aldehyde nickel complex was utilized as an effective activator for
an organosilane in order to generate a hypervalent silicate reactant for
the first time. This method was successfully applied to the highly efficient
syntheses of 3-aryl-, vinyl-, and alkynyl-2,1-benzoxasiloles from benzaldehydes
with aryl-, vinyl-, and alkynylsilyl groups at the ortho position. Initial
mechanistic studies revealed that an intermolecular aryl transfer process
was involved in the reaction mechanism. The formation of an η2-aldehyde
complex was directly confirmed by NMR.
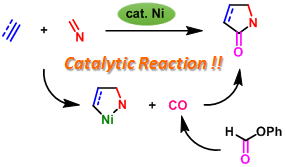
Nickel(0)-Catalyzed [2+2+1] Carbonylative Cycloaddition of Imines and Alkynes or Norbornene Leading to γ-Lactams
Y. Hoshimoto, T. Ohata, Y. Sasaoka, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2014, 136, 15877-15880.
We have developed the first nickel(0)-catalyzed [2+2+1] carbonylative cycloaddition
reaction of imines and alkynes or norbornene by employing phenyl formate
as a CO source. With this method, a variety of N-benzenesulfonyl, -tosyl,
and -phosphoryl-substituted γ-lactams can be prepared in good to
high yields.
Highlight: C&E NEWS 2014, 92, 35. "Refiguring The Equation For [2+2+1] Cycloadditions"
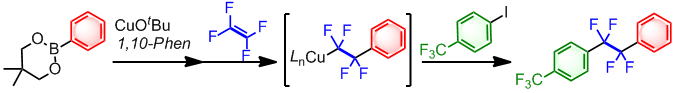
Fluoroalkylcopper(I) Complexes Generated by the Carbocupration of Tetrafluoroethylene: Construction of a Tetrafluoroethylene-Bridging Structure
H. Saijo, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2014, 136, 15158-15161.
We report a copper-mediated synthesis of a variety of 1,2-difunctionalized-1,1,2,2-tetrafluoroethylene
derivatives via the carbocupration of tetrafluoroethylene. The key synthetic
intermediates, 2-aryl-1,1,2,2-tetrafluoroethylcopper complexes, can be
easily prepared, stored, and used as fluoroalkylation reagents.
Highlight: Synfacts (Synfacts 2015, 11, 85)
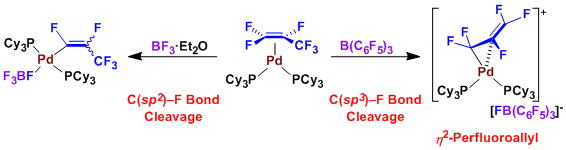
Regioselective C-F Bond Activation of Hexafluoropropylene on Pd(0): Formation of a Cationic η2-Perfluoroallylpalladium Complex
M. Ohashi, M. Shibata, and S. Ogoshi
Angew. Chem. Int. Ed. 2014, 53, 13578.
A chemoselective C(sp2)–F or C(sp3)–F bond activation of hexafluoropropylene (HFP) was achieved by adopting the proper combination of a Lewis acid co-additive with a ligand which coordinates Pd0.
The treatment of [(η2-HFP)Pd(PCy3)2] with B(C6F5)3
allowed a chemoselective C(sp3)–F bond cleavage of HFP to give a cationic perfluoroallypalladium complex. In this complex, the coordination mode of the
perfluoroallyl ligand was considered to be of the unique η2-fashion.
Palladium-Catalyzed Cross-Coupling Reactions of Perfluoro Organic Compounds
M. Ohashi and S. Ogoshi
Catalysts 2014, 4, 321-345.
In this review, we summarize our recent development of palladium(0)-catalyzed cross-coupling reactions of perfluoro organic compounds with organometallic reagents.
Base-Free Hiyama Coupling Reaction via a Group 10 Metal Fluoride Intermediate Generated by C–F Bond Activation
H. Saijo, H. Sakaguchi, M. Ohashi, and S. Ogoshi
Organometallics 2014, 33, 3669-3672. <ACS Editors' Choice>
Group 10 Metal(0)-catalyzed Hiyama coupling reactions of perfluorocompounds were found to proceed without the use of a base.
The key intermediate in these reactions would be a Group 10 Metal(II) fluoride complex that is generated in situ by the
oxidative addition of a C–F bond.
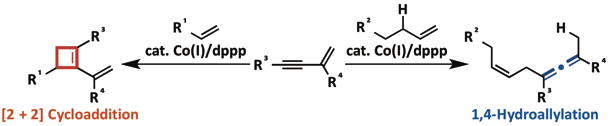
Synthesis of Cyclobutenes and Allenes via Cobalt-Catalyzed Cross-Dimerization of Simple Alkenes with 1,3-Enynes
A. Nishimura, E. Tamai, M. Ohashi, and S. Ogoshi
Chem. Eur. J. 2014, 20, 6613-6617.
In the presence of a Co/dppp catalyst, the [2 + 2] cycloaddition of 1,3-enynes
with alkenes that have no allylic hydrogen (eg. styrene) occurred, while
aliphatic alkenes underwent 1,4-hydroallylation to 1,3-enynes. From simple
substrates, these reactions can provide less-accessible cyclobutenes and
allenes with high chemo- and regio-selective manner.
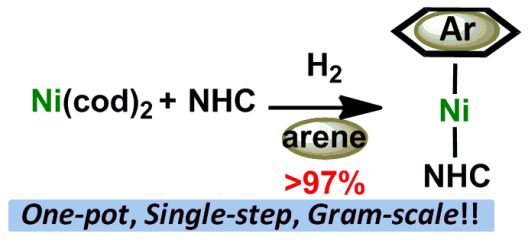
One-Pot, Single-Step and Gram-Scale Synthesis of Mononuclear [(η6-arene)Ni(N-heterocyclic Carbene)] Complexes; Useful Precursors of the Ni0-NHC Unit
Y. Hoshimoto, Y. Hayashi, H. Suzuki, M. Ohashi, and S. Ogoshi
Organometallics 2014, 33, 1276-1282.
Herein we report a one-pot, single-step, and gram-scale method for the
synthesis of [(η6-arene)Ni(NHC)] complexes from commercially available Ni(cod)2 and NHC (or its salt) via hydrogenation of COD. The structure and bonding situations of [(η6-arene)Ni(NHC)]s were evaluated by NMR, X-ray, and DFT studies. An application
to the synthesis of Ni0−NHC complexes via ligand exchange reactions is also demonstrated.
Nickel-Catalyzed Synthesis of N-Aryl-1,2-Dihydropyridines by [2+2+2] Cycloaddition of Imines with Alkynes through T-Shaped 14-Electron Aza-Nickelacycle Key Intermediates
Y. Hoshimoto, T. Ohata, M. Ohashi, and S. Ogoshi
Chem. Eur. J. 2014, 20, 4105-4110.
Nickel-catalyzed synthesis of N-aryl-1,2-dihydropyridines via [2+2+2] cycloadditon
of imines and alkynes has been achieved for the first time by employing
nickel(0)/N-heterocyclic carbene catalyst. In addition, isolation of a
T-shaped 14-electron aza-nickelacycle, which would be a key reaction intermediate,
is also reported.
Palladium-Catalyzed Coupling Reaction of Perfluoroarenes with Diarylzinc Compounds
M. Ohashi, R. Doi, and S. Ogoshi
Chem. Eur. J. 2014, 20, 2040-2048.
The first Pd(0)-catalyzed cross-coupling reaction of hexafluorobenzene (C6F6) with diarylzinc compounds proceeded in the presence of lithium iodide to afford a variety of pentafluorophenyl arenes.
One of the roles of lithium iodide in this catalytic reaction was to promote the oxidative addition of a C−F bond of C6F6 towards palladium.
The other role of lithium iodide was to enhance the reactivity of the organozinc species during the transmetallation step.
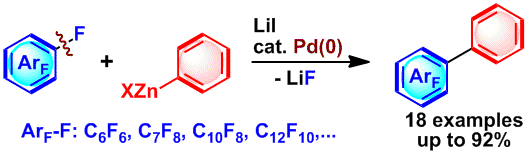
Carbon-Fluorine Bond Activation of Tetrafluoroethylene on Palladium(0) and Nickel(0): Heat or Lewis Acidic Additive Promoted Oxidative Addition
M. Ohashi, M. Shibata, H. Saijo, T. Kambara, and S. Ogoshi
Organometallics 2013, 32, 3631-3639.
Heating both palladium and nickel η2-TFE complexes supported by PCy3 ligands
resulted in C-F bond activation to generate the corresponding trifluorovinyl metal fluorides, trans-(PCy3)2M(F)(CF=CF2).
The C-F bond cleavage of TFE on group 10 metal was found to be accelerated even at room temperature by the addition of not only metal halides,
such as LiI, MgBr2, and AlCl3, but also boron Lewis acids, such as BF3 and B(C6F5)3, giving the corresponding trifluorovinyl metal complexes.

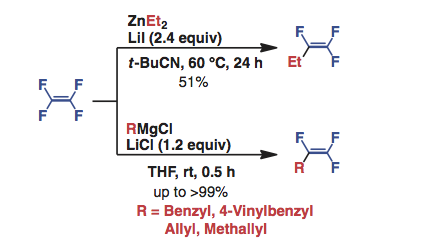
Preparation of Trifluorovinyl Compounds by Lithium Salt Promoted Monoalkylation of Tetrafluoroethylene
M. Ohashi, R. Kamura, R. Doi, and S. Ogoshi
Chem. Lett. 2013, 42, 933-935.
Treatment of tetrafluoroethylene (TFE) with diethylzinc in the presence of lithium iodide gave 1,1,2-trifluoro-1-butene in moderate yield.
Furthermore, in the presence of lithium chloride, the reaction of TFE with benzyl or allyl Grignard reagents afforded the corresponding
monosubstituted products in good to excellent yields.
Nickel-catalyzed [2+2] Cycloaddition Reaction of Bulky Enones with Simple Alkynes. Effect of Bulkiness of Substituent Attached at β-Carbon
A. Abulimiti, A. Nishimura, M. Ohashi, and S. Ogoshi
Chem. Lett. 2013, 42, 904-905.
In the presence of a catalytic amount of Ni(cod)2 and PCy3, the [2+2] cycloaddition of enones bearing a bulky substituent group at β-position with simple alkynes occurs to give cyclobutenes. The bulky group at β-position can suppress the insertion of either an enone to give a cyclohexene or an alkyne to give a cyclohexadiene.
![Nickel-catalyzed [2+2] Cycloaddition Reaction of Bulky Enones with Simple Alkynes](../src/img/publication/2013/img5.png)
Bridging π-coordination of pyrrole and indole over a PdI-PdI bond
T. Murahashi, S. Kimura, K. Takase, S. Ogoshi, and K. Yamamoto
Chem. Commun. 2013, 49, 4310-4312
π-Coordination of pyrrole or indole to a dinuclear PdI-PdI center is revealed. The dinuclear sandwich PdI-PdI complexes of pyrrole, N-methylpyrrole, or indole were synthesized and structurally characterized.
Ni-Catalyzed [4+3+2] Cycloaddition of Ethyl Cyclopropylideneacetate and Dienynes: Scope and Mechanistic Insights
R. Yamasaki, M. Ohashi, K. Maeda, T. Kitamura, M. Nakagawa, K. Kato, T. Fujita, R. Kamura, K. Kinoshita, H. Masu, I. Azumaya, S. Ogoshi, and S. Saito
Chem. Eur. J. 2013, 19, 3415-3425
A detailed study of the Ni-catalyzed [4+3+2] cycloaddition reaction between ethyl cyclopropylideneacetate and dienynes has been conducted, resulting in the development of a new method for the preparation of compounds containing nine-membered rings.
![Ni-Catalyzed [4+3+2] Cycloaddition of Ethyl Cyclopropylideneacetate and Dienynes: Scope and Mechanistic Insights](../src/img/publication/2013/img2.jpg)
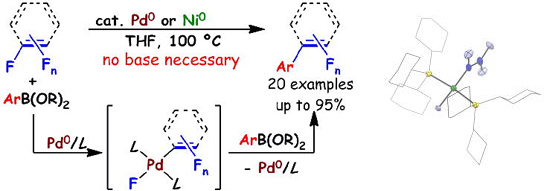
Palladium-Catalyzed Base-Free Suzuki-Miyaura Coupling Reactions of Fluorinated Alkenes and Arenes via a Palladium Fluoride Key Intermediate
M. Ohashi, H. Saijo, M. Shibata, and S. Ogoshi
Eur. J. Org. Chem. 2013, 443-447
A new method for the preparation of trifluorovinyl compounds from tetrafluoroethylene (TFE) and organoboronates has been developed. The key trifluorovinyl palladium(II) fluoride intermediate plays an essential role in this additive-free cross-coupling reaction.
Synthesis of Five- and Six-Membered Benzocyclic Ketones through Intramolecular Alkene Hydroacylation Catalyzed by Nickel(0)/N-Heterocyclic Carbenes
Y. Hoshimoto, Y. Hayashi, H. Suzuki, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2012, 51, 10812-10815
The first example of Ni(0)/NHC-catalyzed intramolecular hydroacylation giving a variety of five- and six-membered benzocyclic ketones is described. The results of mechanistic studies supported the participation of an oxanickelacycle complex in the presented hydroacylation.
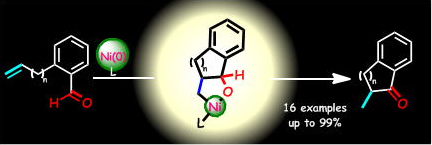
Nickel-Catalyzed Intermolecular [2 + 2] Cycloaddition of Conjugated Enynes with Alkenes
A, Nishimura, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2012, 134, 15692-15695.
We found that conjugated enynes undergo [2 + 2] cycloaddition with alkenes in the presence of Ni(0)/IPr catalyst to give 1-vinylcyclobutenes.
Various electron-deficient alkenes as well as electronically neutral norbornene and α-olefins were applicable to this reaction.
Isolation of the reaction intermediates revealed that the η3-butadienyll coordination is a key for selective formation of cyclobutenes.
![Nickel-Catalyzed Intermolecular [2 + 2] Cycloaddition of Conjugated Enynes with Alkenes](../src/img/publication/2012/img1.jpg)
Selective Construction of Pd2Pt and PdPt2 Triangles in a Sandwich Framework: Carbocyclic Ligands as Scaffolds for a Mixed-Metal System
T, Murahashi, K. Usui, Y. Tachibana, S. Kimura, and S. Ogoshi
Chem. A Eur. J. 2012, 18, 8886-8890.
Redox-induced reversible metal assembly through translocation and reversible ligand coupling in tetranuclear metal sandwich frameworks
T, Murahashi, K. Shirato, A. Fukushima, K. Takase, T. Suenobu, S. Fukuzumi, S. Ogoshi, and H. Kurosawa
Nature Chem. 2012, 4, 52-58.
We report two sandwich complexes in which four palladium centres are incorporated between two π-conjugated ligands, and they exhibit two modes of redox-switchable structural changes. In the first complex, the tetrapalladium chain is split by oxidation into two well-separated dipalladium units. In the second complex, reversible carbon–carbon coupling occurs between the ligands during the redox process.
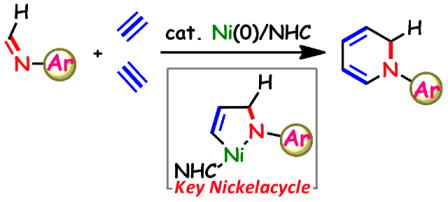
Nickel-catalyzed Dehydrogenative [4 + 2] Cycloaddition of 1,3-Dienes with Nitriles
M. Ohashi, I. Takeda, M. Ikawa, and S. Ogoshi
J. Am. Chem. Soc. 2011, 133, 18018-18021.
We demonstrated the Ni(0)-catalyzed intermolecular dehydrogenative [4 + 2] cycloaddition of 1,3-butadienes with nitriles to give a variety of pyridines regioselectively.
The prepared pyridines in this report were very difficult to prepare by [2 + 2 +2] cycloaddition reactions of alkynes with nitriles.
![Nickel-catalyzed Dehydrogenative [4 + 2] Cycloaddition of 1,3-Dienes with Nitriles](../src/img/publication/2011/img7.jpg)
[3+2] Cycloaddition Reaction of Cyclopropyl Ketones with Alkynes Catalyzed by Nickel/Me2AlCl
T. Tamaki, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2011, 50, 12067-12070.
The nickel-catalyzed [3+2] cycloaddition reaction of cyclopropyl ketones with alkynes gave cyclopentene derivatives in the presence of a catalytic or a stoichiometric amount of dimethylaluminum chloride,
in which organoaluminum reagents activate the carbonyl groups of the cyclopropyl ketones and stabilize the reaction intermediates by the coordination of chloride to nickel.
![[3+2] Cycloaddition Reaction of Cyclopropyl Ketones with Alkynes Catalyzed by Nickel/Me2AlCl](../src/img/publication/2011/img6.jpg)
Nickel-Catalyzed Formation of Cyclopentenone Derivatives via the Unique Cycloaddition of α,β-Unsaturated Phenyl Esters with Alkynes
M. Ohashi, T. Taniguchi, and S. Ogoshi
J. Am. Chem. Soc. 2011, 133, 14900-14903.
We demonstrate the Ni(0)-catalyzed unique [3+2] cycloaddition reaction of α,β-unsaturated phenyl esters with alkynesin iPrOH to yield cyclopentenone derivatives.
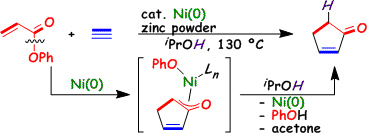
Oxidative Dinuclear Addition of a PdI-PdI Moiety to Arenes: Generation of μ-η3:η3-Arene PdII2 Species
T. Murahashi, K. Takase, M. Oka, and S. Ogoshi
J. Am. Chem. Soc. 2011, 133, 14908-14911.
We report the oxidative dinuclear addition of a PdI-PdI bond to arenes. The oxidative dinuclear addition products which have a bi-π-allyl type arenediyl dipalladium(II) structure were obtained from [2.2]paracyclophane, anthracene, tetracene, and pentacene.
Intra-molecular Oxidative Cyclization of Alkenes and Nitriles with Nickel(0)
M. Ohashi, M. Ikawa, and S. Ogoshi
Organometallics. 2011, 30, 2765-2774.
The use of Me2AlCl as an additive was found to allow the intramolecular oxidative cyclization of 2-(2-methylallyl)benzonitrile on nickel(0) in the presence of PCy3, yielding a nickeladihydropyrrole.
The molecular structures of a series of nickeladihydropyrroles were unambiguously determined by means of X-ray crystallography.
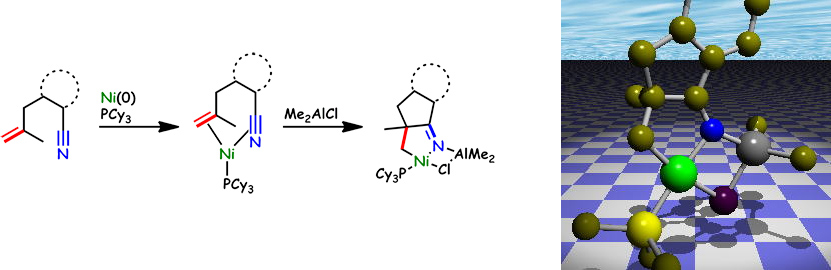
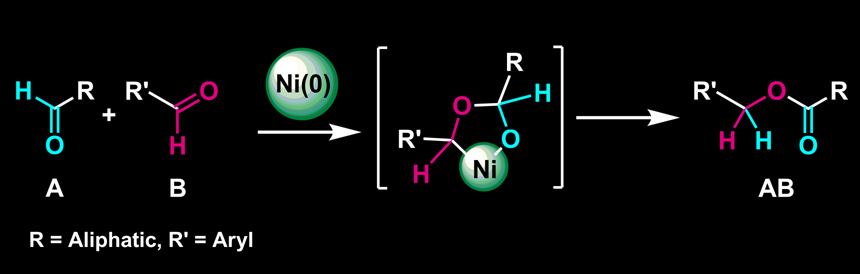
Nickel-Catalyzed Selective Conversion of Two Different Aldehydes to Cross-Coupled Esters
Y. Hoshimoto, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2011, 133, 4668-4671.
In the presence of a Ni(0)/NHC catalyst, an equimolar mixture of aliphatic and aryl aldehydes can be employed to selectively yield a single cross-coupled ester.
This reaction can be applied to a variety of combination of aliphatic and aryl aldehydes.
The reaction represents 100% atom efficiency and generates no waste.
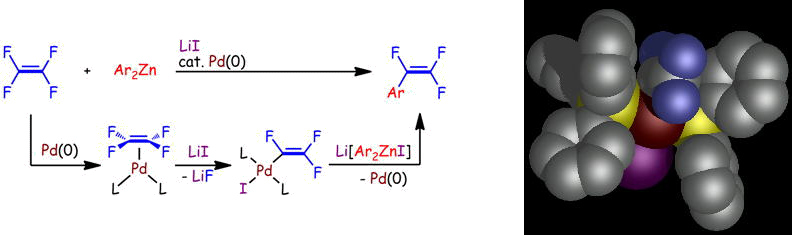
Palladium-catalyzed coupling reactions of tetrafluoroethylene with arylzinc compounds
M. Ohashi, T. Kambara, T. Hatanaka, H. Saijo, R. Doi, and S. Ogoshi
J. Am. Chem. Soc. 2011, 133, 3256-3259.
The first Pd-catalyzed monoarylation of tetrafluoroethylene (TFE) with in situ-prepared diarylzinc reagents yielded α,β,β-trifluorostyrene derivatives in excellent yields with high selectivity.
C-F bond activation of TFE was achieved by the synergetic effects of the Pd(0) species and LiI and generated a trifluorovinyl palladium(II) intermediate.
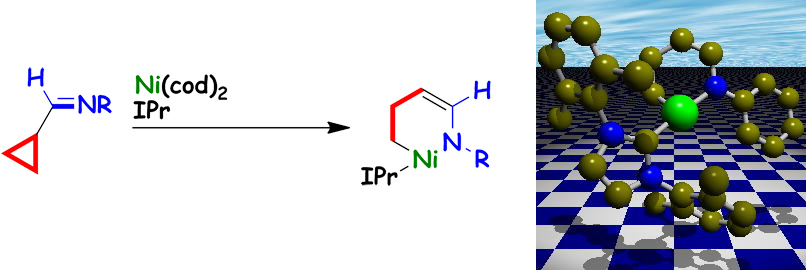
Formation of Six-membered Aza-nickelacycles by Oxidative Addition of Cyclopropyl Imines to Nickel(0)
T. Tamaki, M. Ohashi, and S. Ogoshi
Chem. Lett. 2011, 40, 248-249.
In the presence of IPr, cyclopropyl imines reacted with nickel(0) to give the expected six-membered aza-nickelacycles.
The molecular structure of aza-nickelacyles shows monomeric η1-aza-nickelenolate structures.
The reaction of aza-nickelacycle with an enone occurred to generate an η3-oxa-allylnickel complex.
Formation and coordination modes of the C4E4 moiety (E = COOMe) bound to two palladium(II) moieties
T. Murahashi, T. Okuno, K. Usui, K. Takase, T. Otani, S. Ogoshi, and H. Kurosawa
Dalton Trans. 2011, 40, 2383-2387.
The transmetallation of the palladacyclopentadiene complex Pd{C(COOMe)C(COOMe)C(COOMe)C(COOMe)}(bipy) with the dicationic Pd(II) complex [Pd(bipy)(CH3CN)2][BF4]2 afforded a terminally σ-palladated diene complex [Pd2{μ-η1:η1-C(COOMe)C(COOMe)C(COOMe)C(COOMe)}(bipy)2(CH3CN)2][BF4]2.
Metallocenoids of platinum: Syntheses and structures of triangular triplatinum sandwich complexes of cycloheptatrienyl
T. Murahashi, K. Usui, R. Inoue, S. Ogoshi, and H. Kurosawa
Chem. Sci. 2011, 2, 117-122.
A series of bis-cycloheptatrienyl triangular triplatinum complexes are synthesized as a novel class of organoplatinum sandwich compounds.