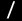Publication
2021-2025 |2016-2020 |2015-2011 | 2010-2006 | 2005-2003
Nickel(0)-Catalyzed Formation of Oxaaluminacyclopentenes via an Oxanickelacyclopentene Key Intermediate:
Me2AlOTf-Assisted Oxidative Cyclization of an Aldehyde and an Alkyne with Nickel(0)
M. Ohashi, H. Saijo, T. Arai, and S. Ogoshi
Organometallics.2010, 29, 6534-6540.
Oxidative cyclization of aldehydes and alkynes with Ni(0) was found to be promoted by addition of Me2AlOTf to yield oxanickelacyclopentenes, which reacted with AlMe3 to be converted the corresponding oxaaluminacyclopentenes.
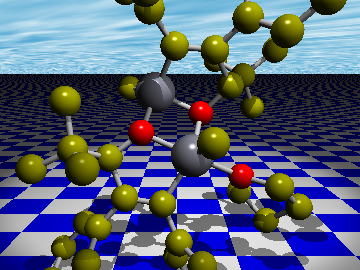
Nickel-catalyzed [2+2+2] Cycloaddition of Two Enones and an Alkyne
S. Ogoshi, A. Nishimura, and M. Ohashi
Org. Lett. 2010, 12, 3450-3452.
A nickel-catalyzed fully intermolecular [2+2+2] cycloaddition of two enones and an alkyne has been developed. In this reaction, one diastereomer was obtained as a sole product.
Nickel/Lewis Acid-Catalyzed Cyanoesterification and Cyanocarbamoylation of Alkynes
Y. Hirata, A. Yada, E. Morita, Y. Nakao, T. Hiyama, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2010, 132, 10070-10077.
Nickel/Lewis acid (LA) cooperative catalysis is found to promote addition reactions of cyanoformates and cyanoformamides across alkynes to give β-cyano-substituted acrylates and acrylamides, respectively, in highly stereoselective and regioselective manners.
Hydrofluoroarylation of Alkynes with Fluoroarenes
K. S. Kanyiva, N. Kashihara, Y. Nakao, T. Hiyama, M. Ohashi and S. Ogoshi
Dalton Trans. 2010, 39, 10483-10494.
In the presence of PCyp3 (Cyp = c-C5H9), Ni(cod)2 is found to be an effective catalyst for chemo-, regio-, and stereo-selective
addition reactions of fluoroarenes across alkynes, giving fluoroaryl ethenes in good to high yields.
[3 + 3] Cyclodimerization of Methylenecyclopropanes: Stoichiometric and Catalytic Reactions of Nickel(0) with Electron-Deficient Alkylidenecyclopropanes
M. Ohashi, T. Taniguchi and S. Ogoshi
Organometallics 2010, 29, 2386-2389
Stoichiometric treatment of Ni(cod)2 with ethyl cyclopropylideneacetate (ECPA) in the presence of PCy3 resulted in an unpredicted formation of a Ni(0) complex bearing an
(E,E)-1,2-bis(exo-alkylidene)cyclohexane ligand, which stemmed from the [3 + 3] cyclodimerization of ECPA .The reaction could be expanded to a Ni(0)-catalyzed [3 + 3] cyclodimerization reaction of ester-substituted
methylenecyclopropanes, giving the corresponding cyclohexane derivatives in excellent yields.
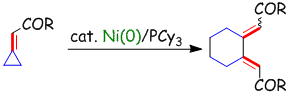
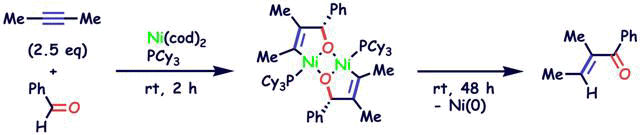
Nickel-catalyzed Tishchenko reaction via hetero-nickelacycles by oxidative cyclization of aldehydes with nickel(0) complex
S. Ogoshi, Y. Hoshimoto and M. Ohashi
Chem. Commun. 2010, 46, 3354-3356
A Ni(0)-catalyzed Tishchenko reaction which can be applied to a variety of aliphatic and aromatic aldehydes was developed. The reaction might proceed via a hetero-nickelacycle intermediate.
Nickel-catalyzed Reactions between Enone and Two Ethylenes
S. Ogoshi, A. Nishimura, T. Haba and M. Ohashi
Chem. Lett. 2009, 38, 1166-1167
In the presence of a catalytic amount of Ni(cod)2 and PCy3, the reaction of (E)-1-phenylbut-2-en-1-one with ethylene occurred to give three-component
addition products, 1,6-enone and 1,5-diketone. The former product was obtained from the reaction of an enone with two ethylenes, whereas the latter was obtained from the reaction of two enones with an ethylene.
2.gif)
Nickel-Catalyzed Direct Conjugate Addition of Simple Alkenes to Enones
S. Ogoshi, T. Haba and M. Ohashi
J. Am. Chem. Soc. 2009, 131, 10350-10351
We report a direct conjugate addition of simple alkenes to enones by the addition of a C-H bond. Although conjugate additions generally require organometallic reagents and the employed metals must be discarded as waste,
no metal salt waste is generated in this reaction catalyzed by a common nickel/phosphine complex.
.gif)
Square Tetrapalladium Sheet Sandwich Complexes: Cyclononatetraenyl as a Versatile Face-Capping Ligand
T. Murahashi, R. Inoue, K. Usui, S. Ogoshi
J. Am. Chem. Soc. 2009, 131, 9888-9889.
An unusual square tetrapalladium sheet sandwich complex, [Pd4(μ4-C9H9)(μ4-C8H8)][B(Arf)4]
has been isolated and structurally characterized. X-ray structure analysis showed that the square palladium sheet is flanked by the nine-membered cyclononatetraenyl and eight-membered cyclooctatetraene ligands to form a square metal sheet molecular sandwich structure.
.gif)
Ni(0)-Catalyzed Formation of Azaaluminacyclopentenes via Azanickelacyclopentenes: A Unique Nickel/Aluminum Double Transmetalation Reaction
M. Ohashi, O. Kishizaki, H. Ikeda and S. Ogoshi
J. Am. Chem. Soc. 2009, 131, 9160-9161.
Aza-aluminacyclopentenes, which serve a useful precursor for ganma-substituted allylamines, were catalytically prepared from novel Ni-mediated three-component cyclocondensation of an alkyne, an imine, and AlMe3.
.gif)
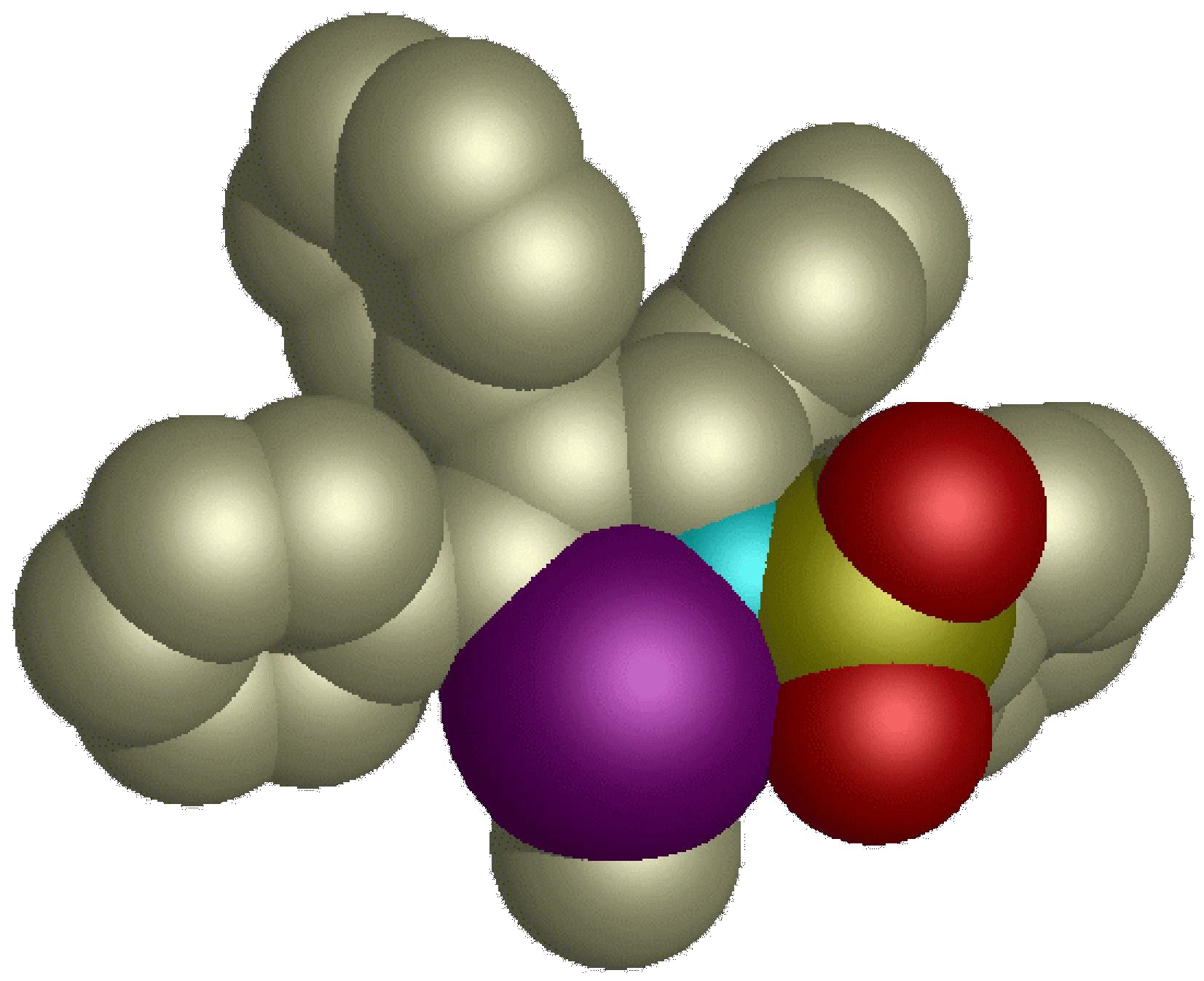
Synthesis and Reactivity of Six-Membered Oxa-Nickelacycles: Ring-Opening Reaction of Cyclopropyl Ketones
T. Tamaki, M. Nagata, M. Ohashi and S. Ogoshi
Chem. Eur. J. 2009, 15, 10083-10091.
In the presence of IPr, cyclopropyl phenyl ketone rapidly undergoes oxidative addition to nickel(0) to give a six-membered oxa-nickelacycles. The molecular structure shows a monomeric η1-nickelenolate structure,
and the coordination geometry of the nickel(II) center (formally 14-electron) is regarded as "an unusual T-shaped planar".
2.gif)
.gif)
Intramolecular Arylcyanation of Alkenes Catalyzed by Nickel/AlMe2Cl
Y. Nakao, S. Ebata, A. Yada, T. Hiyama, M. Ikawa, and S. Ogoshi
J. Am. Chem. Soc. 2008, 130, 12874-12875.
A catalyst system derived from Ni(cod)2 and cocatalytic AlMe2Cl effects the intramolecular arylcyanation of alkenes.
Nickel-catalyzed [2+2+2] cycloaddition of two alkynes and an imine
S. Ogoshi, H. Ikeda, and H. Kurosawa
Pure Appl. Chem. 2008, 80, 1115-1125.
In the presence of 10 mol% of Ni(cod)2 and PMetBu2 at 100 deg, the intermolecular [2+2+2] cycloaddition of N-benzylidenebenzenesulfonamide and 2-butyne occurred to give the expected 1,2-dihydropyridine in 87 % yield.
Structures of Two Haptotropic Isomers Generated by the Sliding of 1,3,5-Triene Ligands on a Pd-Pd-Pd Chain
T. Murahashi, Y. Mino, K. Chiyoda, S. Ogoshi, and H. Kurosawa
Chem. Commun. 2008, 4061-4063.
Two haptotropic isomers of [Pd3(μ3-DMVC)2(CH3CN)2][BF4]2
(DMVC = 1,2-di-(E)-methoxyvinylcyclopentene) were structurally determined by X-ray crystallographic analyses.
Reductive Coupling of Metal Triangles in Sandwich Complexes
T. Murahashi, Y. Hashimoto, K. Chiyoda, M. Fujimoto, T. Uemura, R. Inoue, S. Ogoshi, and H. Kurosawa
J. Am. Chem. Soc. 2008, 130, 8586-8587.
The one-electron reduction of [Pd3(C7H7)2(CH3CN)3][BF4]2
in acetonitrile resulted in the formation of the dicationic Pd3-Pd3 dimer [Pd6(C7H7)4(CH3CN)4][BF4]2.
Formation of Acylruthenium Promoted by Coordination of AlMe3 to (η4-Cyclopentadienone)Ru(CO)3
S. Ogoshi, T. Kato, M. Ohashi, and H. Kurosawa
Dalton Trans. 2008, 2232-2234.
Treatment of (η4-tetraphenylcyclopentadienone)Ru(CO)3 with AlMe3 yields an adduct arising from coordination of the enone oxygen to aluminium.
The adduct undergoes alkylation of the Ru(CO)3 moiety to give (η5-C4Ph4C(OAlMe2))Ru(CO)2(COMe) quantitatively.
Synthesis and structure of dipalladium complexes containing cyclooctatetraene and bicyclooctatrienyl ligands
T. Murahashi, N. Kato, S. Ogoshi, and H. Kurosawa
J. Organomet. Chem 2008, 693, 894-898.
The reaction of a substitutionally labile dipalladium(I) complex [Pd2(CH3CN)6][BF4]2
with 1,3,5,7-cyclooctatetraene in acetonitrile affords [Pd2(η3:η3-C8H8)(CH3CN)4][BF4]2.
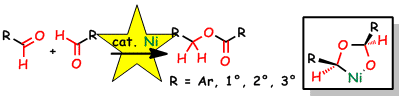
Nickeladihydrofuran. Key Intermediate for Nickel-catalyzed Reaction of Alkyne and Aldehyde
S. Ogoshi, T. Arai, M. Ohashi, and H. Kurosawa
Chem. Commun. 2008, 1347-1349.
We demonstrated the formation of a nickeladihydrofuran by oxidative cyclization of an alkyne and an aldehyde in the presence of nickel(0).
Nickeladihydrofuran is suggested to be an important key intermediate in a variety of catalytic reactions.
A stable zerovalent palladium chain enveloped by a pi-electron sheath of conjugated polyene ligands
Y. Tatsumi, T. Murahashi, M. Okada, S. Ogoshi, and H. Kurosawa
Chem. Commun. 2008, 477-479.
A stable homoleptic Pd(0)4 chain complex supported by non-activated olefins was isolated and structurally characterized by X-ray diffraction study, and the unique structure and
bonding are compared to those of the corresponding dicationic [Pd4]2+ chain complex.
Discrete triangular tripalladium sandwich complexes of arenes
T. Murahashi, M. Fujimoto, Y. Kawabata, R. Inoue, S. Ogoshi, and H. Kurosawa
Angew. Chem. Int. Ed. 2007, 46, 5440-5443.
A Bis([2.2]paracyclophane)tripalladium complex, [Pd3([2.2]paracyclophane)2(MeCN)3][BArF]2
(BArF = B[3,5-(CF3)2C6H3]4), was isolated and characterized by NMR spectroscopy,
and a mixed-ligand derivative was structurally characterized.
Formation of an Aza-nickelacycle by Reaction of an Imine and an Alkyne with Nickel(0): Oxidative Cyclization, Insertion, and Reductive Elimination
S. Ogoshi, H. Ikeda, and H. Kurosawa
Angew. Chem. Int. Ed. 2007, 46, 4930-4932.
The oxidative cyclization of an imine and an alkyne in the presence of nickel(0) followed by the insertion of a second alkyne affords a seven-membered
aza-nickelacycle. Subsequent reductive elimination gives a 1,2-dihydropyridine. This sequential reaction is expanded to a one-pot nickel-catalyzed [2+2+2]
cycloaddition of two alkynes and an imine.
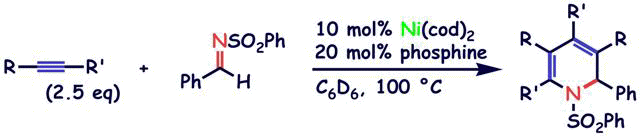
Rearrangement of a Pd4 skeleton from a 1D chain to a 2D sheet on the face of a perylene or fluoranthene ligand caused by exchange of the binder molecule.
T. Murahashi, N. Kato, T. Uemura, and H. Kurosawa
Angew. Chem. Int. Ed. 2007, 46, 3509-3512.
A chain-to-sheet rearrangement of the Pd4 moiety is observed during the exchange of one perylene or fluoranthene ligand of a bis(perylene) or a bis(fluoranthene) tetra-palladium sandwich complex by 1,3,5,7-cyclooctatetraene.
Sandwich complexes containing bent palladium chains
Y. Tatsumi, K. Shirato, T. Murahashi, S. Ogoshi, and H. Kurosawa
Angew. Chem. Int. Ed.2006, 45, 5799-5803.
Treatment of the bis(perylene)tetrapalladium complex [Pd4(perylene)2(CH3CN)2][BArF]2
with 5 equiv of 1,2-bis(4-phenyl-1,3- butadienyl)benzene (o-BPBB) resulted in the quantitative formation of the bis(o-BPBB)tetrapalladium complex [Pd4(o-BPBB)2][BArF]2.
Discrete Sandwich Compounds of Monolayer Palladium Sheets
T. Murahashi, M. Fujimoto, M.-a. Oka, Y. Hashimoto, T. Uemura, Y. Tatsumi, Y. Nakao, A. Ikeda, S. Sakaki, and H. Kurosawa
Science 2006, 313, 1104-1107.
Two multinuclear sandwich complexes, a triangular Pd3 complex wedged between two cycloheptatrienyl ligands and an edge-sharing
triangle-trapezoid-shaped Pd5 sheet supported by two naphthacene rings, were prepared.
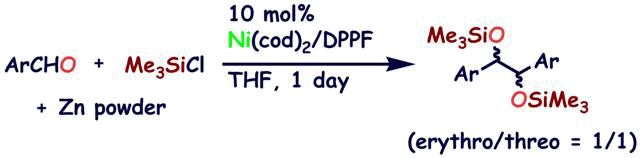
Reaction of (η2-arylaldehyde)nickel(0) complexes with Me3SiX (X = OTf, Cl). Application to catalytic reductive homocoupling reaction of arylaldehyde
S. Ogoshi, H. Kamada, and H. Kurosawa
Tetrahedron 2006, 62, 7583-7588.
Arylaldehydes can coordinate to Ni(0) in η2-fashion to give (η2-arylaldehyde)nickel complexes,
which react with Me3SiOTf or Me3SiCl to give (η1:η1-siloxybenzyl)nickel
or (η3-siloxybenzyl)nickel complex. In the presence of PCy3 or CO, the (η1:η1-siloxybenzyl)nickel
complex underwent homocoupling reaction, yielding a pinacol type product.
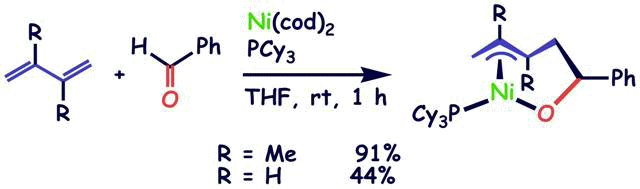
Reversible Carbon-Carbon Bond Formation between 1,3-Dienes and Aldehyde or Ketone on Nickel(0)
S. Ogoshi, K.-I. Tonomori, M.-a. Oka, and H. Kurosawa
J. Am. Chem. Soc. 2006, 128, 7077-7086.
Treatment of 2,3-R2-1,3-butadiene with aldehydes R1CHO in the presence of [Ni(cod)2(PCy3)]
underwent the reversible oxidative cyclization to afford η3:η1-3-pentenyloxy nickel complexes
[[(3,4,5-η)-CH2CR2CR2CH2CH(R1)O]Ni(PCy3)].
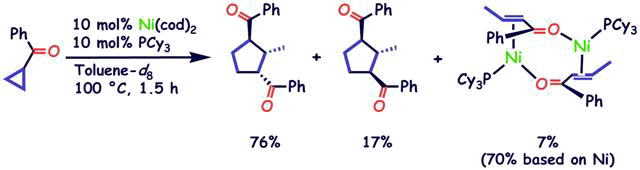
Formation of Nickeladihydropyran by Oxidative Addition of Cyclopropyl Ketone. Key Intermediate in Nickel-Catalyzed Cycloaddition
S. Ogoshi, M. Nagata, and H. Kurosawa
J. Am. Chem. Soc. 2006, 128, 5350-5351
Cyclopropyl phenyl ketone underwent oxidative addition to a Ni(0) species, yielding a nickeladihydropyran. This is a key intermediate for the Ni(0)-catalyzed homo- or heterocycloaddition
to give cyclopentane compounds having two carbonyl substituents at the 1,3-position.
Dinuclear addition of the Pd-Pd moieties to 1,3-dienes
T. Murahashi, T. Nagai, H. Nakashima, S. Tomiyasu, and H. Kurosawa
Chem. Lett. 2006, 35, 754-755
The dinuclear palladium complex [Pd2(μ-η3:η1-C5H8)(CH3CN)5][BF4]2
was prepared from the reaction of a substitutionally labile dipalladium(I) complex [Pd2(CH3CN)6][BF4]2 with isoprene.
Stereoretentive elimination and trans-olefination of the dicationic dipalladium moiety [Pd2Ln]2+ Bound on 1,3,5-Trienes
T. Murahashi, H. Nakashima, T. Nagai, Y. Mino, T. Okuno, M. A. Jalil, and H. Kurosawa
J. Am. Chem. Soc. 2006, 128, 4377-4388
The reaction of [Pd2(CH3CN)6][BF4]2 with
1,3,5-hexatriene (HT), 1,6-diphenyl-1,3,5-hexatriene (DPHT), or 2,2,9,9-tetramethyl-3,5,7-decatriene (DBHT) gave the corresponding bi-eta3-allyldipalladium complexes
[Pd2(μ-η3:η3-HT)(CH3CN)4][BF4]2,
[Pd2(μ-η3:η3-DPHT)(CH3CN)4][BF4]2,
and [Pd2(μ-η3:η3-DBHT)(CH3CN)4][BF4]2.
Primary metal chain complexes
T. Murahashi and H. Kurosawa
Kagaku Furontia, 2006, 16, 55-62