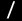Cleavage of C(sp3)–F Bonds in Trifluoromethylarenes Using a Bis(NHC)nickel(0) Complex
H. Iwamoto, H. Imiya, M. Ohashi, S. Ogoshi
J. Am. Chem. Soc., 2020, 142, 19360-19367.
The oxidative addition of a single C(sp3)–F bond in trifluoromethylarenes to a transition-metal complex is described.
A nickel(0) complex that bears two NHC ligands is able to cleave the C(sp3)–F
bonds. Isolation and characterization studies suggested that
the cleavage of
the C(sp3)−F bond proceeds via a η2-arene
nickel(0) complex. Taking advantage of the reactivity, we
developed a catalytic
hydrodefluorination of trifluoromethylarenes. A computational
study indicated
that the electron-rich nickel(0) center supported by two NHC
ligands cleaves
the C(sp3)–F bond via a syn-SN2ʹ mechanism.
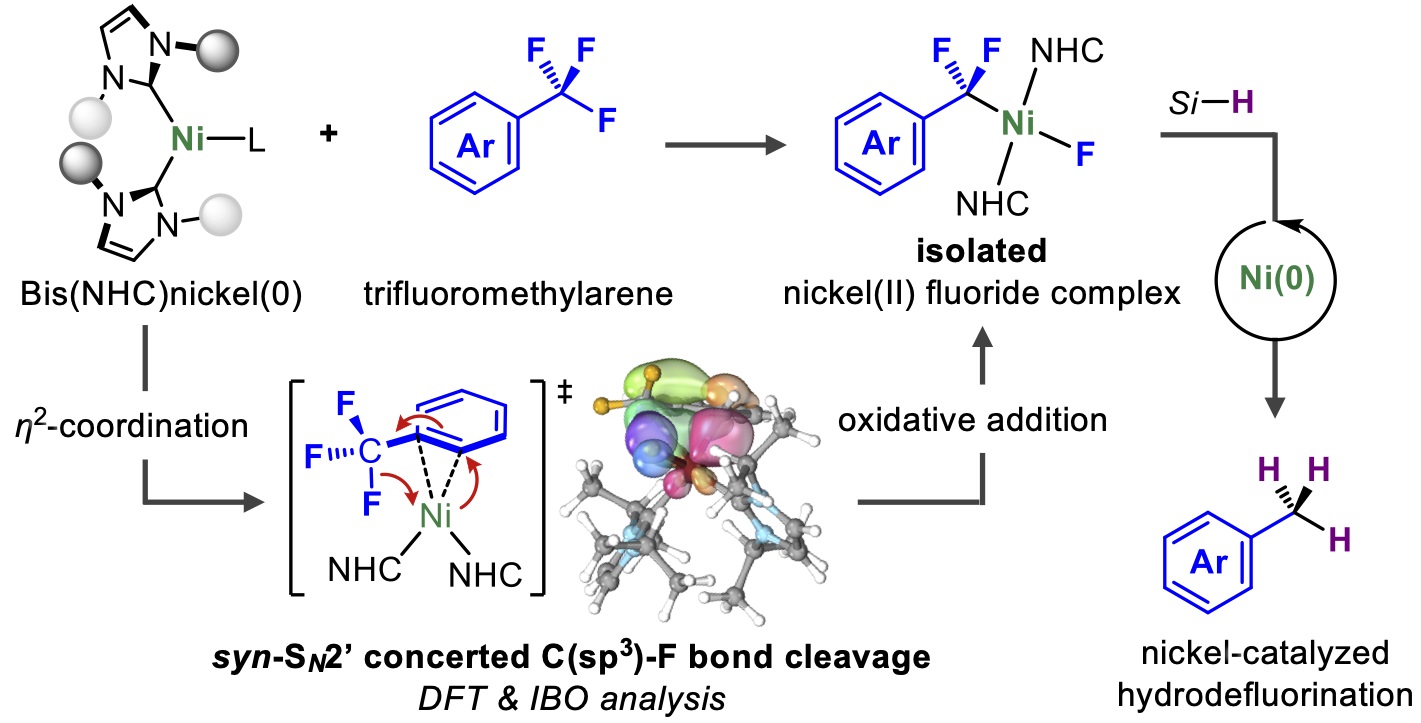
CsF-Catalyzed Fluoroacylation of Tetrafluoroethylene Using Acyl Fluorides for the Synthesis of Pentafluoroethyl Ketones
N. Ishida, H. Iwamoto, D. E. Sunagawa, M. Ohashi, S. Ogoshi
Synthesis, 2021, 53, 3137-3143.
A catalytic method for the synthesis of pentafluoroethyl ketones has been
developed. The cesium fluoride catalyst can be used to convert acyl fluorides
into the pentafluoroethyl ketones under tetrafluoroethylene pressure without
generating stoichiometric quantities of chemical waste. Mechanistic studies
suggest that high reaction temperature is crucial for the ketone to be
the major product..
Invited Contribution to "Bond Activation - in Honor of Prof. Shinji
Murai"
Ni(0)-Catalyzed Synthesis of Polycyclic α,β-Unsaturated γ-Lactams via Intramolecular Carbonylative Cycloaddition of Yne-imines with CO
K. Ashida, Y. Hoshimoto, S. Ogoshi
Synlett, 2021, 32, 1537-1541.
A Ni(0)-catalyzed intramolecular carbonylative cycloaddition between 1,5-yne-imines
and carbon monoxide (CO) is disclosed. When Ni(CO)3PCy3 was employed as a pre-catalyst, a variety of polycyclic α,β-unsaturated
γ-lactams were prepared in up to 78% yield with 100% atom efficiency. Aza-nickelacycles,
generated by the oxidative cyclization of yne-imines on the Ni(0) center,
were experimentally confirmed as key intermediates. Moreover, diastereoselective
transformations of the obtained products to afford highly substituted polycyclic
γ-lactams with three contiguous carbon stereocenters are reported.
Invited Contribution to "Modern Nickel-Catalyzed Reactions"
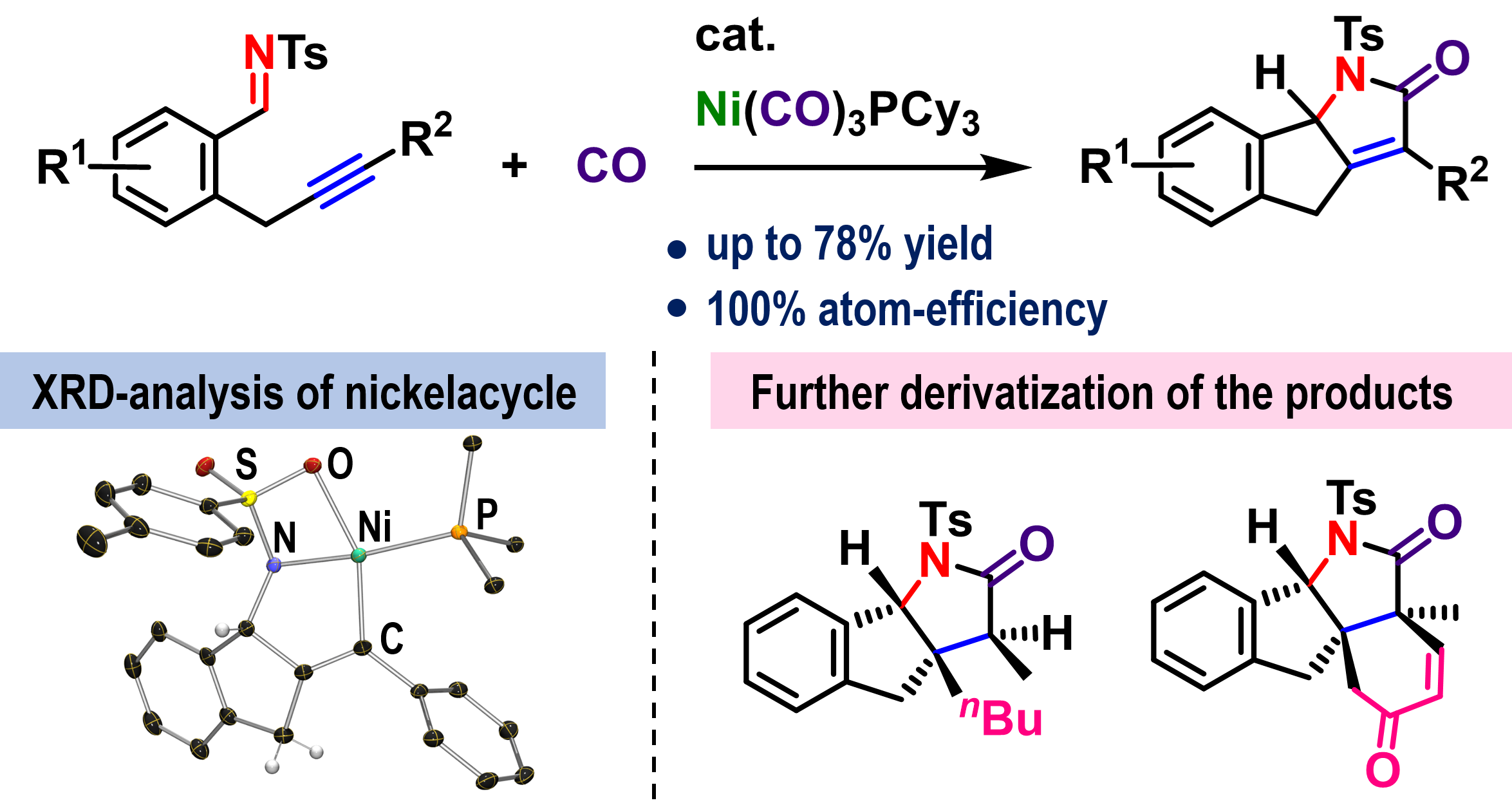
Direct Transformation of Tetrafluoroethylene to Trifluorovinylzinc via sp2 C–F Bond Activation
K. Kikushima, Y. Etou, R. Kamura, I. Takeda, H. Ito, M. Ohashi, S. Ogoshi
Org. Lett., 2020, 22, 8167-8172.
Here, we report a novel synthetic approach for the formation of trifluorovinylzinc chloride
via a C–F bond activation of tetrafluoroethylene (TFE), which is an industrially
cost-effective bulk feedstock with a negligible GWP. The present system provides
a practical synthetic route to various trifluorovinyl derivatives with very low energy consumption.
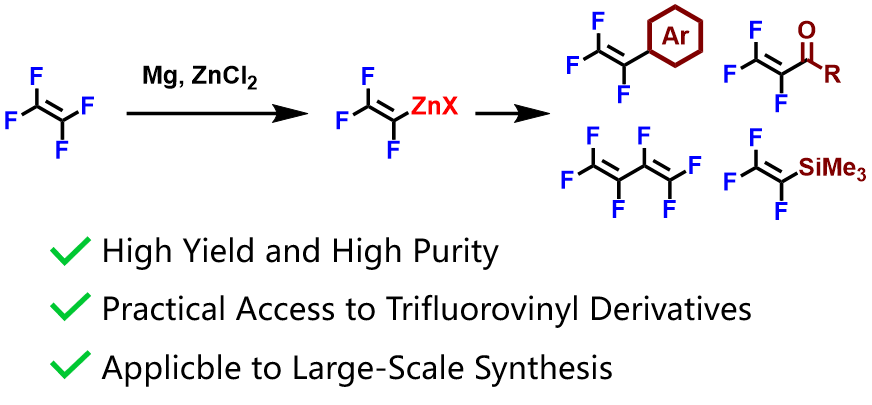
Rotation-Triggered Transmetalation on Heterobimetallic Cu/Al N-Phosphine-Oxide-Substituted Imidazolylidene Complex
T. Asada, Y. Hoshimoto, and S. Ogoshi
J. Am. Chem. Soc., 2020, 142, 9772-9784.
A novel strategy for the preparation of heterobimetallic N-heterocyclic carbene (NHC) complexes is demonstrated using N-phosphine-oxide-substituted imidazolylidenes (PoxIms). In these heterobimetallic Cu/Al complexes, the Cu and Al centers can be either completely separated or brought near each other via the rotation of the N-phosphinoyl group in the PoxIm ligands. The present results demonstrate the role of phosphine oxide in the activation of organoaluminum reagents for the transmetalation between Cu(I) complexes bearing NHCs, as well as the benefit of constructing an intramolecular system based on a heterobimetallic complex to achieve efficient transmetalation by programming the encounter of two organometallic fragments.
OPEN ACCESS
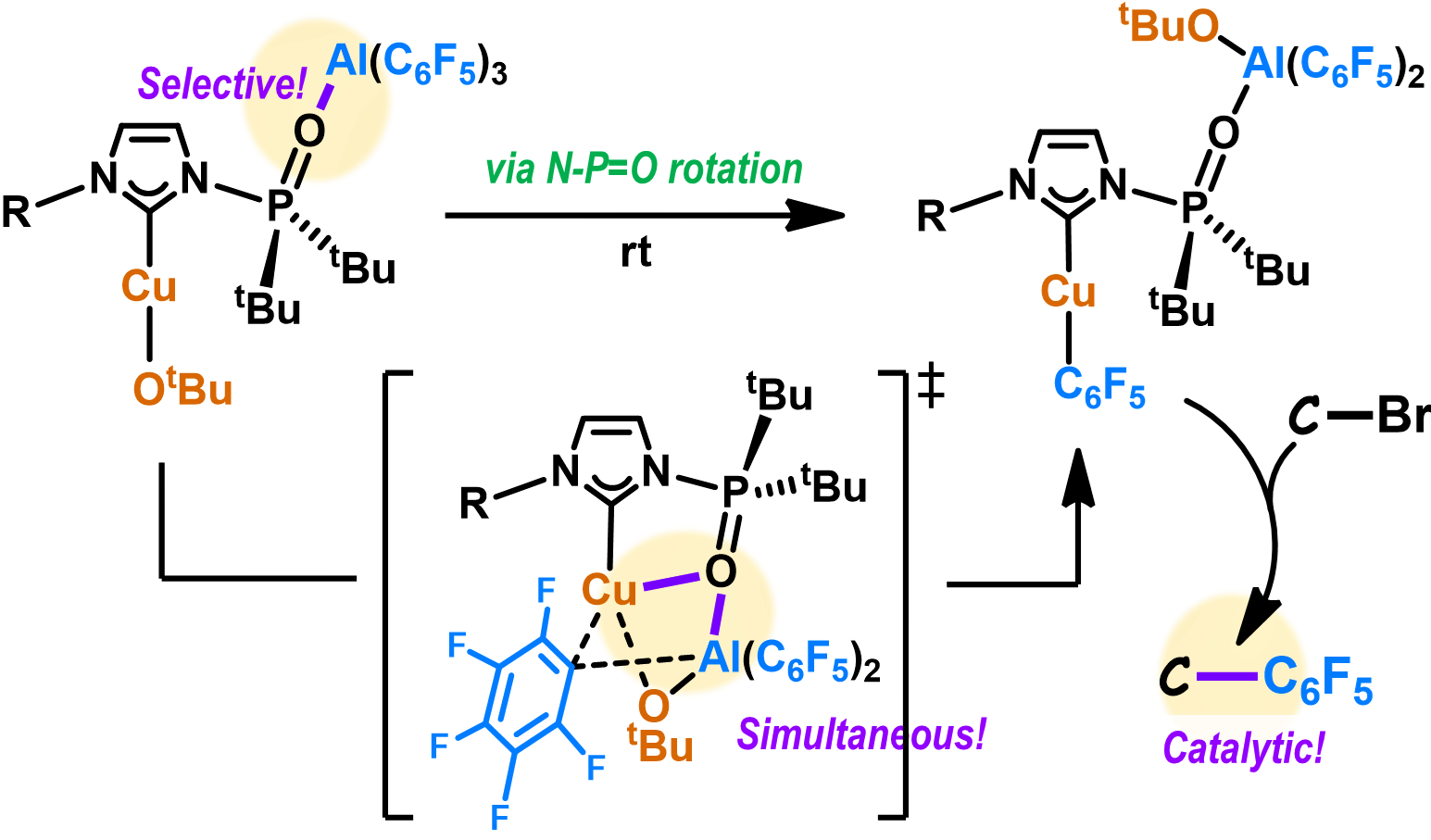
Axial Chirality around N-P Bonds Induced by Complexation betwen E(C6F5)3 (E = B, Al) and an N-Phosphine-Oxide-Substituted Imidazolinylidene: A Key Intermediate in the Catalytic Phosphinoylation of CO2
T. Asada, Y. Hoshimoto, T. Kawakita, T. Kinoshita and S. Ogoshi
J. Org. Chem., 2020, 85, 14333-14341.
Complexation-induced axial chirality around an N‒P bond occurs upon the
predominant coordination of the N-phosphinoyl group in the N-phosphine-oxide-substituted
imidazolinylidene (SPoxIm) to B(C6F5)3. Conversely, this axial chirality was not certainly observed via the complexation between SPoxIm and Al(C6F5)3. The carbene carbon atoms in (κ-O-SPoxIm)E(C6F5)3 (E = B, Al) remain sufficiently nucleophilic to react with CO2.
OPEN ACCESS; One of the Most Read Articles
Special issue "The New Golden Age of Organophosphorus Chemistry"
Enantioselective Synthesis of Polycyclic γ-Lactams with Multiple Chiral Carbon Centers via Ni(0)-Catalyzed Asymmetric Carbonylative Cycloadditions without Stirring
K. Ashida, Y. Hoshimoto, N. Tohnai, D. Scott, M. Ohashi, H. Imaizumi, Y.
Tsuchiya and S. Ogoshi
J. Am. Chem. Soc., 2020, 142, 1594-1602.
Herein, we report a strategy to construct polycyclic γ-lactam derivatives that contain more than two contiguous stereogenic centers in an enantioselective as well as atom-economic manner. The results of mechanistic studies, including the isolation of a chiral heteronickelacycle, support that the enantioselectivity on the two contiguous carbon atoms of the γ-lactams is determined during the oxidative cyclization on nickel(0).
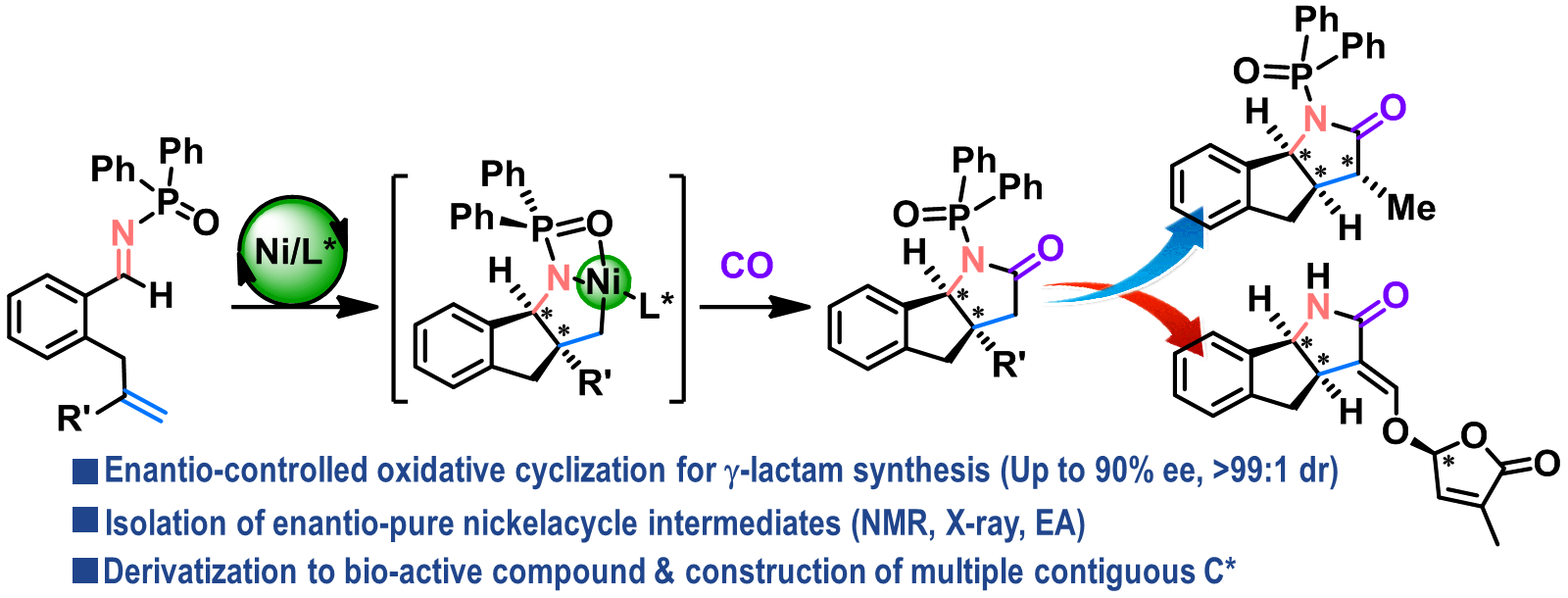
Catalytic Synthesis of Isoquinolines via Intramolecular Migration of N-Aryl Sulfonyl Groups on 1,5-Yne-Imines
Y. Hoshimoto, C. Nishimura, Y. Sasaoka, R. Kumar and S. Ogoshi
Bull. Chem. Soc. Jpn, 2020, 93, 182-186.
Isoquinolines are ubiquitous structural motifs in a variety of bioactive
compounds, including medicinal agents and natural products. Herein, we
report a simple amine-catalyzed protocol for the synthesis of isoquinolines
from 1,5-yne-imines via the intramoleular migration of an N-aryl sulfonyl group to the carbon atom of the alkyne moiety.
OPEN ACCESS
Nickel Catalysis in Organic Stnthesis: Methods and Reactions
Edited by S. Ogoshi, Wiley-VCH, 2020.

Nickel-catalyzed decarbonylation of N-acylated N-heteroarenes
T. Morioka, S. Nakatani, Y. Sakamoto, T. Kodama, S. Ogoshi, N. Chatani,
M. Tobisu
Chem. Sci. 2019, 10, 6666-6671.
Triarylborane-Catalyzed Reductive N-Alkylation of Amines: A Perspective
Y. Hoshimoto, and S. Ogoshi
ACS Catal. 2019, 9, 5439-5444.
Recent progress on the triarylborane (BAr3)-catalyzed reductive N-alkylation of amines is briefly summarized in this Perspective. Key aspects
on several catalytic systems are emphasized in order to discuss the reaction
mechanisms, substrate scope, and practical applications for the preparation
of medicinal compounds, as well as the identification of remaining challenges
in this area.
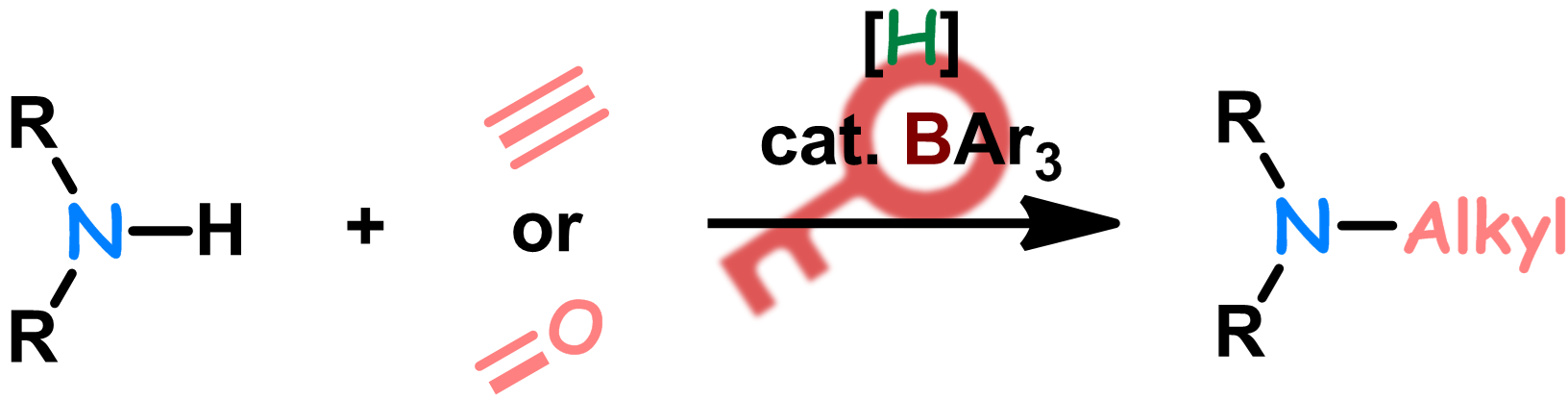
Ni(0)-Catalyzed Three-Component Coupling Reaction of Tetrafluoroethylene and N-Sulfonyl-Substituted Imines with Silanes via Aza-Nickelacycles
H. Shirataki, T. Ono, M. Ohashi, and S. Ogoshi
Org. Lett., 2019, 21, 851-856.
A nickel-catalyzed three-component coupling reaction of tetrafluoroethylene (TFE) and N--sulfonyl-substituted imines with silanes that
furnishes a variety of fluorine-containing amines is disclosed. Stoichiometric experiments revealed that the aza-nickelacycles generated via
oxidative cyclization of TFE and N--sulfonyl-substituted imines on Ni(0) were identified as the key intermediates in this catalytic reaction.
A single-crystal X-ray diffraction study on such an aza-nickelacycle revealed that the O atom of the N--sulfonyl group stabilizes the key
intermediate via coordination to the nickel center.
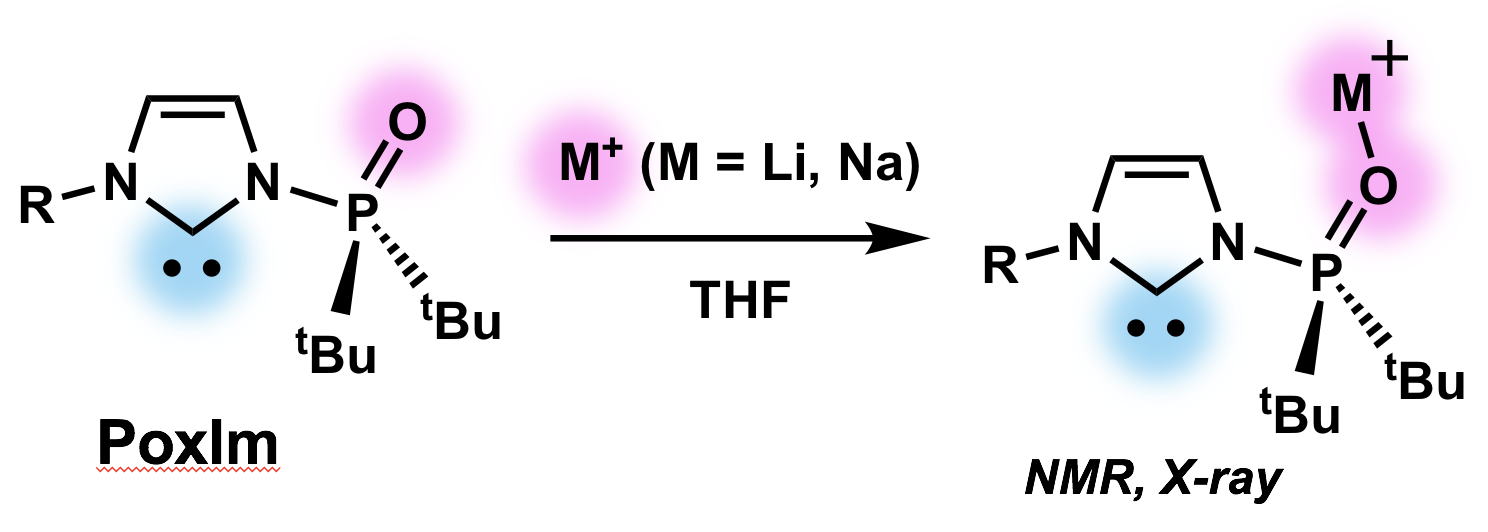
Complexation between MOTf (M = Li and Na) and N-Phosphine Oxide-substituted Imidazolylidenes via Coordination of the N-Phosphoryl Groups
T. Kinoshita, M. Sakuraba, Y. Hoshimoto, and S. Ogoshi
Chem. Lett., 2019, 48, 230-233.
A reaction between an N-phosphine oxide-substituted imidazolylidene (PoxIm) and MOTf (M = Li or Na) in THF afforded
[(PoxIm)x(MOTf)y(THF)z]
through the coordination of the N-phosphoryl group to Na+ or Li+, which was unambiguously confirmed by 13C and 31P NMR spectroscopy and single-crystal X-ray diffraction analyses.
OPEN ACCESS
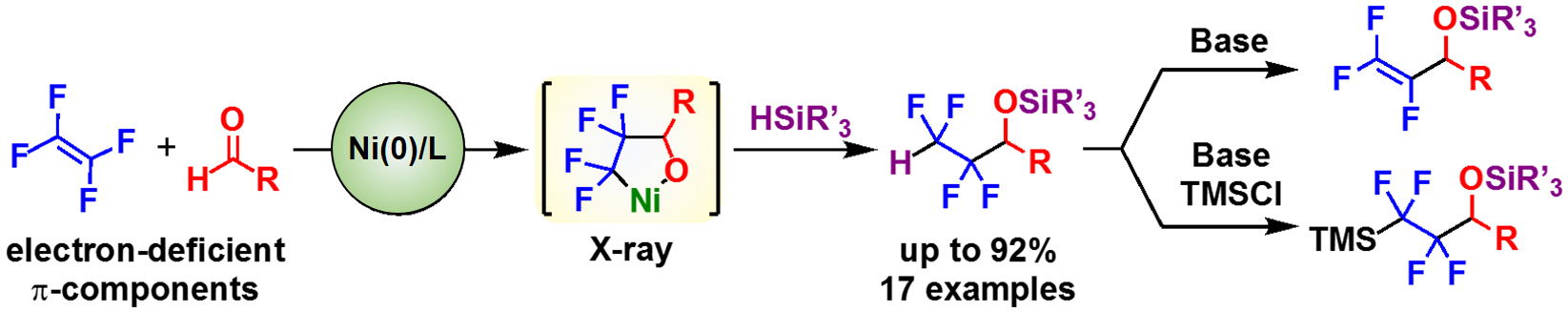
Nickel-catalyzed Three-component Coupling Reaction of Tetrafluoroethylene and Aldehydes with Silanes via Oxa-Nickelacycles
H. Shirataki, M. Ohashi, and S. Ogoshi
Eur. J. Org. Chem., 2019, 9, 1883-1887.
The nickel-catalyzed synthesis of a variety of fluorine-containing silyl ethers from tetrafluoroethylene (TFE)
and aldehydes with silanes in a selective manner is disclosed. Stoichiometric reactions revealed that the oxa-nickelacycle,
which is generated upon oxidative cyclization of TFE and an aldehyde with Ni(0), is the key intermediate, whose molecular
structure was confirmed by single-crystal X-ray diffraction analysis.
Highlighted as a Cover Picture
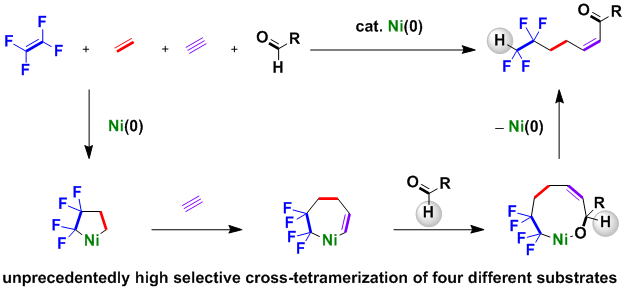
Selective Catalytic Formation of Cross-Tetramers from Tetrafluoroethylene, Ethylene, Alkynes, and Aldehydes via Nickelacycles as Key Reaction Intermediates
T. Kawashima, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc., 2018, 140, 17423-17427.
In the presence of a catalytic amount of Ni(cod)2 (cod = 1,5-cyclooctadiene)
and PCy3 (Cy = cyclohexyl), the cross-tetramerization of tetrafluoroethylene (TFE), ethylene,
alkynes, and aldehydes leads to a variety of fluorine-containing enone derivatives. The present catalytic system produces a solo cross-tetramer in a highly
selective manner from more than 256 (= 4^4) any combination of four different unsaturated compounds.
Strain-Induced Double Carbon-Carbon Bond Activations of Cycloparaphenylenes by a Platinum Complex: Application to the Synthsis of Cyclic Diketones
E. Kayahara, T. Hayashi, K. Takeuchi, F. Ozawa, K. Ashida, S. Ogoshi, S.
Yamago
Angew. Chem. Int. Ed.. 2018, 57, 11418-11421.
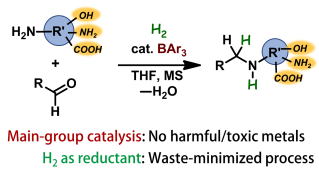
Main-Group-Catalyzed Reductive Alkylation of Multiply Substituted Amines with Aldehydes Using H2
Y. Hoshimoto, T. Kinoshita, S. Hazra, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2018, 140, 7292-7300.
A novel reductive alkylation system using H2 is presented, which proceeds via a tandem reaction that involves the B(2,6-Cl2C6H3)(p-HC6F4)2-catalyzed formation of an imine and the subsequent hydrogenation of this
imine catalyzed by a frustrated Lewis pair. Press Release, 日経バイオテクONLINE, 化学工業(2018, 69, 551-552), academist Journal, Chem-Station, EurekAlert, AlphaGalileo, PhysOrg, ScienceDaily.
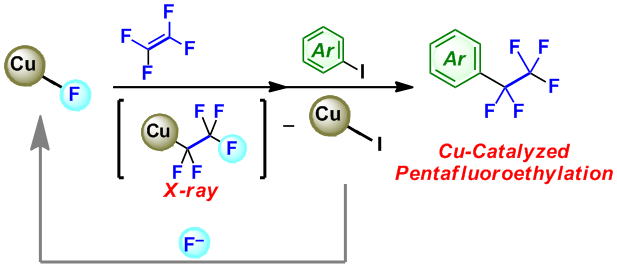
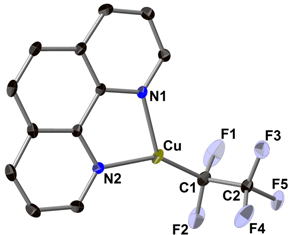
Cu(I)-Catalyzed Pentafluoroethylation of Aryl Iodides in the Presence of Tetrafluoroethylene and Cesium Fluoride: Determining the Route to Key Pentafluoroethyl Cu(I) Intermediate
M. Ohashi, N. Ishida, K. Ando, Y. Hashimoto, A. Shigaki, K. Kikushima, and S. Ogoshi
Chem. Eur. J. 2018, 24, 9794-9798.
The Cu(I)‐catalyzed pentafluoroethylation of iodoarenes via the fluorocupration of tetrafluoroethylene (TFE) is disclosed. The active species, (phen)CuC2F5, was isolated and its molecular structure confirmed by a single‐crystal X‐ray diffraction analysis.
The key to the successful suppression of the competing oligomerization of TFE is to refrain from stirring the reaction mixture.
A mechanistic study clearly discarded the possibility that the catalytic reaction proceeds via a radical pathway.
Highly Atom Economical Molecular Transformation via Hetero-Nickelacycle
S. Ogoshi
Bull. Chem. Soc. Jpn. 2017, 90, 1401-1406
This account includes the formation of nickelacycles from nickel(0) species and development of catalytic reactions without using coupling reagents
to construct highly atom economical nickel-catalyzed multicomponent connecting reactions.
OPEN ACCESS
Award Account
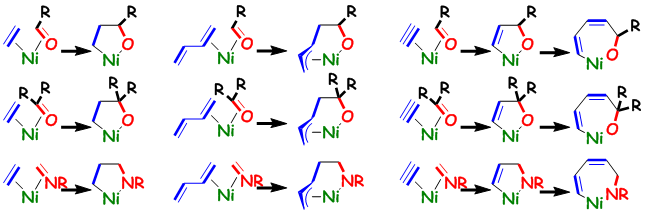
Nickel-Catalyzed Formation of 1,3-Dienes via a Highly Selective Cross-Tetramerization of Tetrafluoroethylene, Styrenes, Alkynes, and Ethylene
T. Kawashima, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc. 2017, 139, 17795-17798.
In the presence of a catalytic amount of Ni(cod)2 and PCy3, the cross-tetramerization of tetrafluoroethylene (TFE), alkynes,
and ethylene occurred in a highly selective manner to afford a variety of 1,3-dienes with a 3,3,4,4-tetrafluorobutyl chain.
In addition, a Ni(0)-catalyzed cross-tetramerization of TFE, alkynes, ethylene, and styrene was developed. These catalytic
reactions might proceed via partially fluorinated five- and seven-membered nickelacycle key intermediates.
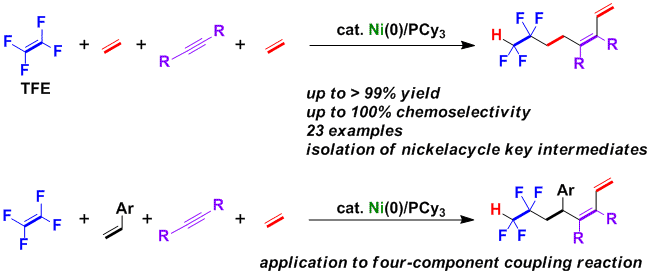
Fluorinated Vinylsilanes from the Copper-Catalyzed Defluorosilylation of Fluoroalkene Feedstocks
H. Sakaguchi, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2018, 57, 328-332.
A copper-catalyzed C-F bond defluorosilylation reaction of fluoroalkene feedstocks such as tetrafluoroethylene and other polyfluoroalkenes is developed.
Mechanistic studiesrevealed that the key steps of this defluorosilylation reaction are: i) the 1,2-addition of a silylcopper intermediate to the polyfluoroalkene,
and ii) a subsequent selective β-fluorine elimination, which generates a Cu-F species. The β-fluorine elimination is facilitated by the Lewis acidic F-Bpin,
which is generated in situ during the defluorosilylation.
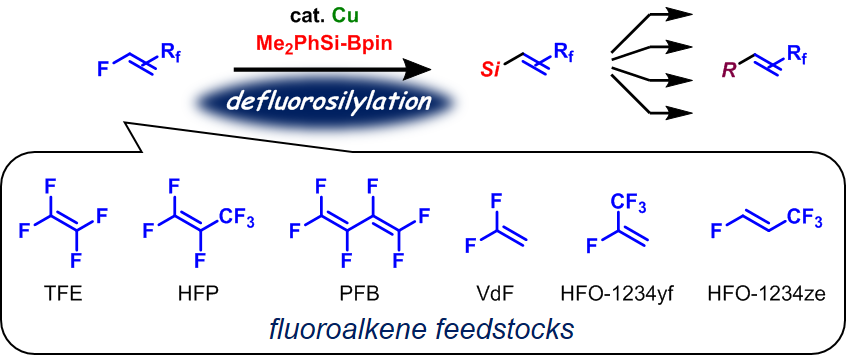
Transition-metal-free catalytic hydrodefluorination of polyfluoroarenes via a concerted nucleophilic aromatic substitution with a hydrosilicate
K. Kikushima, M. Grellier, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2017, 56, 16191-16196.
The transition-metal-free catalytic hydrodefluorination (HDF) reaction of polyfluoroarenes is developed, which involves a direct hydride transfer
from a hydrosilicate as the key intermediate, generated from a hydrosilane and a fluoride salt. The eliminated fluoride regenerates the hydrosilicate to complete
the catalytic cycle. Dispersion-corrected density functional theory (DFT) calculations indicated that the HDF reaction proceeds via a concerted nucleophilic
aromatic substitution (CSNAr) reaction, whereby a Meisenheimer-type intermediate is not generated.
N-Phosphine Oxide-Substiuted Imidazolylidenes (PoxIms): Multifunctional Multipurpose Carbenes
S. Hazra, Y. Hoshimoto, S. Ogoshi
Chem. Eur. J. 2017, 23, 15238-15243.
This article discusses the concept of N-heterocyclic carbenes (NHCs) equipped with more than one functionality, which allow using these NHCs for multiple purposes. A pioneering example for such NHCs is N-phosphine oxide-substituted imidazolylidenes (PoxIms), and their synthesis
and strategic use are highlighted. The utility of PoxIms exceeds by far
the conventional use as multidntate ligands for metal complexes on account
of the synergetic functions of the carbene and the N-phosphine oxide group(s).
OPEN ACCESS
Invited 'Concept' Article; Most Accessed article; Highlited in Frontispiece
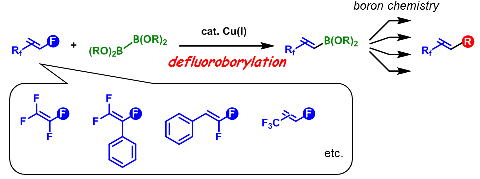
Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes en Route to Diverse Fluoroalkenes
H. Sakaguchi, Y. Uetake, M. Ohashi, T. Niwa, S. Ogoshi, and T. Hosoya
J. Am. Chem. Soc. 2017, 139, 12855-12862.
Monodefluoroborylation of polyfluoroalkenes, such as tetrafluoroethylene, (trifluorovinyl)arenes, (difluorovinyl)arenes, and trifluoromethylated monofluoroalkenes,
has been achieved in a regioselective manner under mild conditions via copper catalysis. Stoichiometric experiments indicate that the fate of the regioselectivity
depends on the mode of β-fluorine elimination, which depends on the substrate.
Press Release
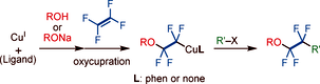
Synthesis and Reactivity of Fluoroalkyl Copper Complexes by the Oxycupration of Tetrafluoroethylene
M. Ohashi, T. Adachi, N. Ishida, K. Kikushima, and S. Ogoshi
Angew. Chem. Int. Ed. 2017, 56, 11911-11915.
The copper(I)-mediated generation of -OCF2CF2-
moieties by the oxycupration of tetrafluoroethylene (TFE) using either copper aryloxides or alkoxides is disclosed. The key intermediates,
2-aryloxy-1,1,2,2-tetrafluoroethyl and 2-alkoxy-1,1,2,2-tetrafluoroethyl copper complexes, were obtained from the reaction of the corresponding
aryloxy and alkoxy copper complexes with TFE, and their structures in solution and in the solid state were unambiguously determined by
multinuclear NMR spectroscopy and X-ray diffraction analysis. These copper complexes subsequently reacted with aryl iodides (ArI) to afford
ROCF2CF2Ar (R=aryl or alkyl) in high yields.
Hot Paper
Highlighted as a Back Cover Picture
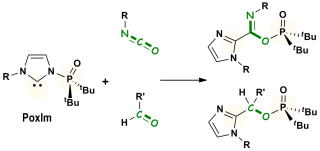
Phosphorylation of Isocyanates and Aldehydes Mediated by Multifunctional N-Phosphine Oxide-Substituted Imidazolylidenes
Y. Hoshimoto, T. Asada, S. Hazra, M. Ohashi, and S. Ogoshi
Chem. Lett. 2017, 46, 1211-1213.
The direct syntheses of imidic-phosphinic mixed anhydrides and phosphinates
were accomplished via phosphorylation of isocyanates or aldehydes with
N-phosphine oxide-substitued imidazolylidenes (PoxIms) that are equipped
with both nucleophilic carbene and electrophilic phosphorus moieties.
OPEN ACCESS
Nickel(0)-Catalyzed Coupling Reactions of Carbonyls and Alkenes with Reducing Reagents Giving Six- and Seven-Membered Benzocycloalkanols
Y. Hayashi, Y. Hoshimoto, K. Ravindr,. M. Ohashi, and S. Ogoshi
Chem. Lett. 2017, 46, 1096-1098.
Nickel(0)-catalyzed coupling reactions of 1,6- and 1,7-enals with triethylsilane
have been utilized to synthesize six- and seven-membered silyl-protected
benzocycloalkanols. When triethylborane was employed, tetralol derivatives
were obtained.
OPEN ACCESS
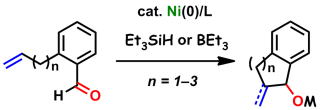
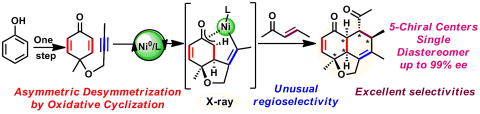
Two-step synthesis of chiral fused tricyclic scaffolds from phenols via desymmetrization on nickel
R. Kumar, Y. Hoshimoto, E. Tamai, M. Ohashi, and S. Ogoshi
Nat. Commun. 2017, 8, 32.
Here we show a nickel-catalyzed highly enantioselective synthesis of hydronaphtho[1,8-bc]furans with five contiguous chiral centers via desymmetrization of alkynyl-cyclohexadienone by oxidative cyclization and following formal [4 + 2] cycloaddition processes. Alkynyl-cyclohexadienone was synthesized in one step from easily accessible phenols. This reaction represents excellent chemo-selectivity, regio-selectivity, diastereo-selectivity, and enantio-selectivity (single diastereomer, up to 99% ee).
OPEN ACCESS; Press Release; Highlighted in 日刊工業新聞(6月30日), Nature Japan, Chem-Station, AlphaGalileo
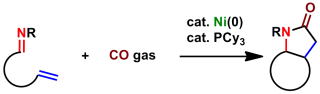
Efficient Synthesis of Polycyclic γ-Lactams by Catalytic Carbonylation of Ene-Imines via Nickelacycle Intermediate
Y. Hoshimoto, K. Ashida, Y. Sasaoka, R. Kumar, K. Kamikawa, X. Verdaguer,
A. Riera, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2017.56, 8206-8210.
CO gas is employed in a nickel(0)-catalyzed [2+2+1] carbonylative cycloaddition reaction with 1,5- and 1,6-ene-imine substrates. The method affords a variety of tri- and tetracyclic γ-lactams in excellent yields with 100 % atom efficiency.
Most Accessed article
Copolymerisation of ethylene with polar monomers by using palladium catalysts bearing an N-heterocyclic carbene-phosphine oxide bidentate ligand
W. Tao, S. Akita, R. Nakano, S. Ito, Y. Hoshimoto, S. Ogoshi and K. Nozaki
Chem. Commun. 2017, 53, 2630-2633.
We report the synthesis and characterization of palladium complexes bearing
an N-heterocyclic carbene-phosphine oxide bidantate ligand and their use
as catalysts for ethylene polymerization and ethylene/polar monomer copolymerization.
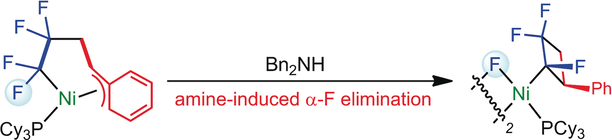
Nickel(0)-Mediated Transformation of Tetrafluoroethylene and Vinylarenes into Fluorinated Cyclobutyl Compounds
M. Ohashi, Y. Ueda and S. Ogoshi
Angew. Chem. Int. Ed. 2017, 56, 2435-2439.
In the presence of Ni(0)/PCy3,
styrene was found to participate in oxidative cyclization with
tetrafluoroethylene, thus leading to the corresponding nickelacycle with
a unique η3-π-benzyl structure. In addition, the flexibility of the coordination mode in the η3-benzyl
moiety allowed the partially fluorinated nickelacycle to undergo
unprecedented amine-induced α-fluorine elimination, thus leading to the
construction of a fluorinated cyclobutyl skeleton.
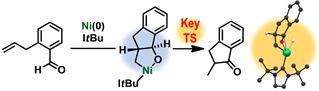
Kinetic and Theoretical Studies on Ni(0)/N-Heterocyclic Carbene-Catalyzed Intramolecular Alkene Hydroacylation
Y. Hoshimoto, Y. Hayashi, M. Ohashi, and S. Ogoshi
Chem. Asian J. 2017, 12, 278-282.
A monomeric and trans-nickelacycle intermediate was found to be key for
occurance of the rate-limiting β-hydride elimination in Ni(0)/ItBu-catalyzed
intramolecular alkene hydroacyaltion, which was revealed by a combined
kinetic and theoretical study.
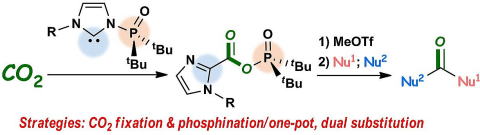
Strategic Utilization of Multifunctional Carbene for Direct Synthesis of Carboxylic-Phosphinic Mixed Anhydride from CO2
Y. Hoshimoto, T. Asada, S. Hazra, T. Kinoshita, P. Sombut, R. Kumar, M.
Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2016, 55, 16075-16079.
Strategic utilization of N-phosphine oxide-substituted imidazolylidenes
enabled a direct preparation of a novel carboxylic-phosphinic mixed anhydride
from carbon dioxide, which was followed by efficient derivatization into
a variety of carbonyl compounds.
OPEN ACCESS
Transition-Metal Mediated Transformations of Tetrafluoroethylene into Various Polyfluorinated Organic Compounds
M. Ohashi, and S. Ogoshi
有機合成化学協会誌 (J. Synth. Org. Chem., Jpn.), 2016, 74, 1047-1057.
This account describes our studies on the transition-metal mediated transformation
of tetrafluoroethylene into a variety of polyfluorinated organic compounds,
manifesting merits of a derivatization of readily available perfluorinated
compounds to valuable highly fluorinated organic compounds.
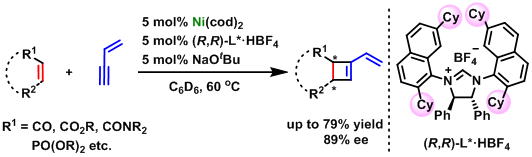
Nickel-Catalyzed Enantioselective Synthesis of Cyclobutenes via [2+2] Cycloaddition of α,β-Unsaturated Carbonyls with 1,3-Enynes
R. Kumar, E. Tamai, A. Ohnish, A. Nishimura, Y. Hoshimoto, M. Ohashi,
and S. Ogoshi
Synthesis. 2016, 48, 2789-2794.
A nickel(0)-catalyzed enantioselective approach has been developed for
[2+2] cycloaddition of alkenes with conjugated enynes for the first time.
A new chiral NHC was synthesized to yield cyclobutenes in good yields and
enantioselectivities.
Special Topic
Highlighted as a Front Cover Picture
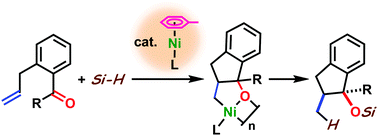
Ni(0)-catalyzed intramolecular reductive coupling of alkenes and aldehydes or ketones with hydrosilane
Y. Hayashi, Y. Hoshimoto, R. Kumar, M. Ohashi, and S. Ogoshi
Chem. Commun. 2016, 52, 6237-6240.
A nickel(0)-catalyzed reductive coupling of aldehydes and simple alkenes
with hydrosilanes has been developed. The present system was also applied
to a reductive coupling with ketones. Preliminary results of a nickel(0)-catalyzed
asymmetric three-component coupling reaction of an aldehyde, an alkene,
and triethylsilane are also shown.
OPEN ACCESS
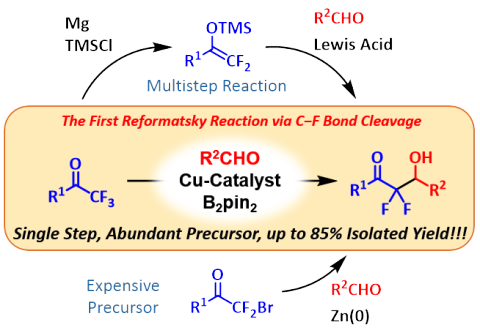
Copper-Catalyzed Reaction of Trifluoromethylketones with Aldehydes via a Copper Difluoroenolate
R. Doi, M. Ohashi, and S. Ogoshi
Angew. Chem. Int. Ed. 2016, 55, 341-344.
A copper-catalyzed reaction of easily accessible α,α,α-trifluoromethylketones
with various aldehydes affords difluoro-methylene compounds in the presence
of diboron and NaOtBu. The key process of the reaction is the formation of a copper difluoroenolate by 1,2-addition of a borylcopper intermediate to α,α,α-trifluoromethylketones and subsequent
β-fluoride elimination.
Highlight: Synfacts (Synfacts 2016, 12, 78)