home > Pablication
2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
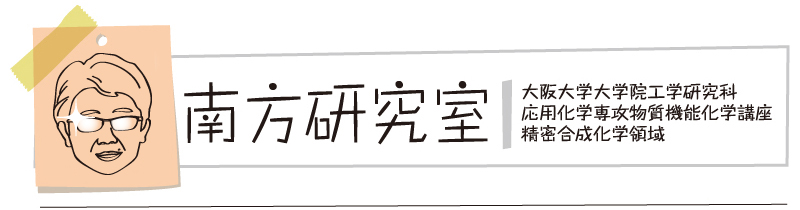
“On-surface synthesis of nitrogen-doped nanographene with an [18]annulene pore on Ag(111)"
Kewei Sun, Donglin Li, Takahiro Kaihara, Satoshi Minakata, Youhei Takeda*, and Shigeki Kawai*
Commun. Chem. 2023, 6, 228/1–7. DOI:10.1038/s42004-023-01023-z
*Open-access article!
*Invited as a part of a Special Collection on "On-surface synthesis"!
Abstract: On-surface synthesis is of importance to fabricate low dimensional carbon-based nanomaterials with atomic precision. Here, we synthesize nitrogen-doped nanographene with an [18]annulene pore and its dimer through sequential reactions of debromination, aryl–aryl coupling, cyclodehydrogenation and C–N coupling on Ag(111) from 3,12-dibromo-7,8-diaza[5]helicene. The inner structures of the products were characterized with scanning tunneling microscopy with a CO terminated tip at low temperature. Furthermore, the first four unoccupied electronic states of the nanographene were investigated with a combination of scanning tunneling spectroscopy and theoretical calculations. Except for the LUMO + 2 state observed at +1.3 V, the electronic states at 500 mV, 750 mV and 1.9 V were attributed to the superatom molecular orbitals at the [18]annulene pore, which were significantly shifted towards the Fermi level due to the hybridization with the confined surface state.
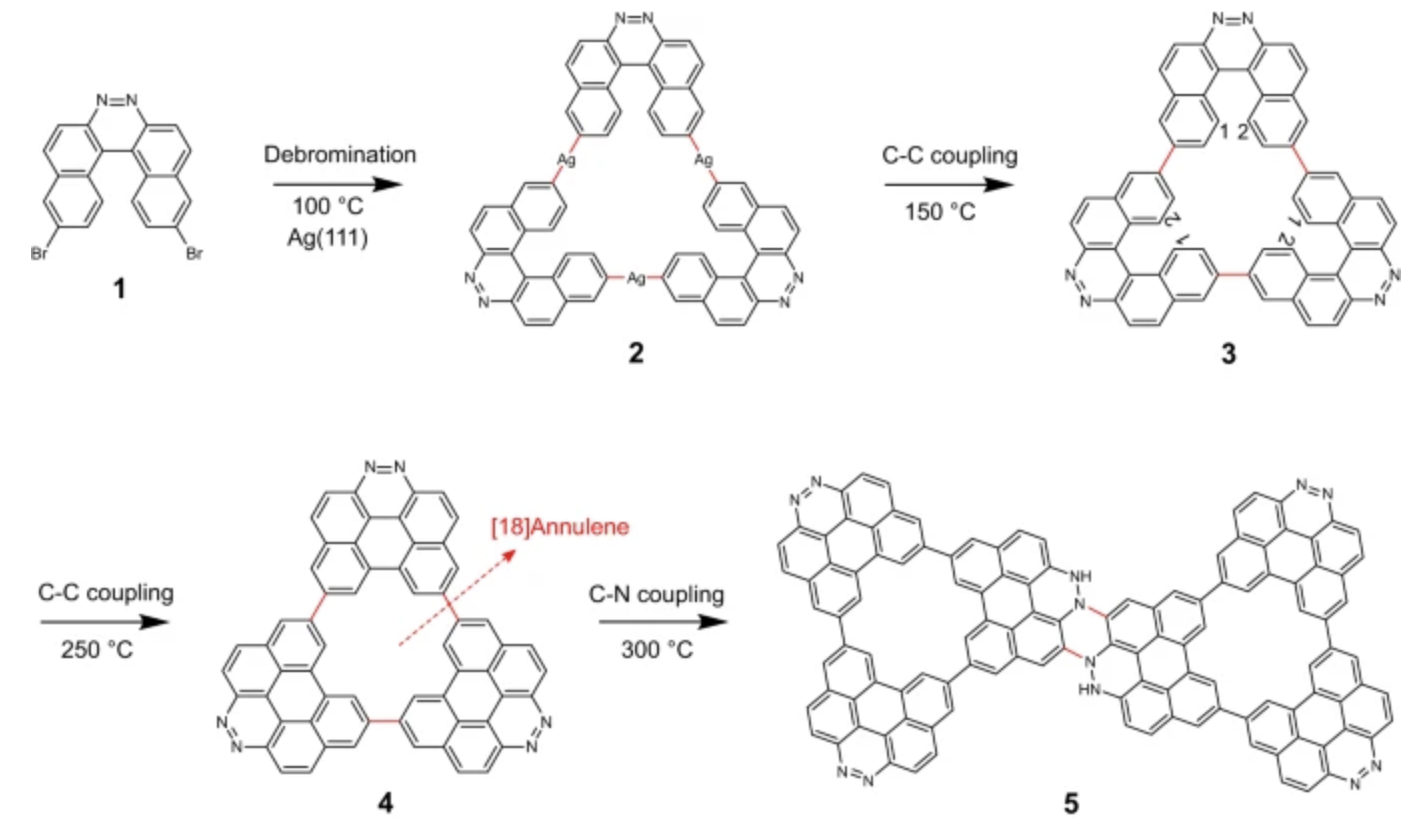
“Femtosecond Spectroscopy on a Dibenzophenazine-Cored Macrocycle Exhibiting Thermally Activated Delayed Fluorescence"
Kirstoffer A. Thom, Oliver Nolden, Oliver Weingart*, Saika Izumi, Satoshi Minakata, Youhei Takeda*, and Peter Gilch*
ChemistryOpen 2023, 12, e202300026/1–10. DOI:10.1002/open.202300026
*Open-access article!
*Invited as a part of a Special Collection on "Emissive Materials for Organic Light Emitting Diodes"!
Abstract: Using steady-state and transient absorption spectroscopy as well as quantum chemical calculations, the depicted TADF macrocycle is investigated. It features strongly solvent-dependent S1 lifetimes and concomitantly triplet yields. While embedded in matrix environments the TADF character is evident, in solution these properties are lost.
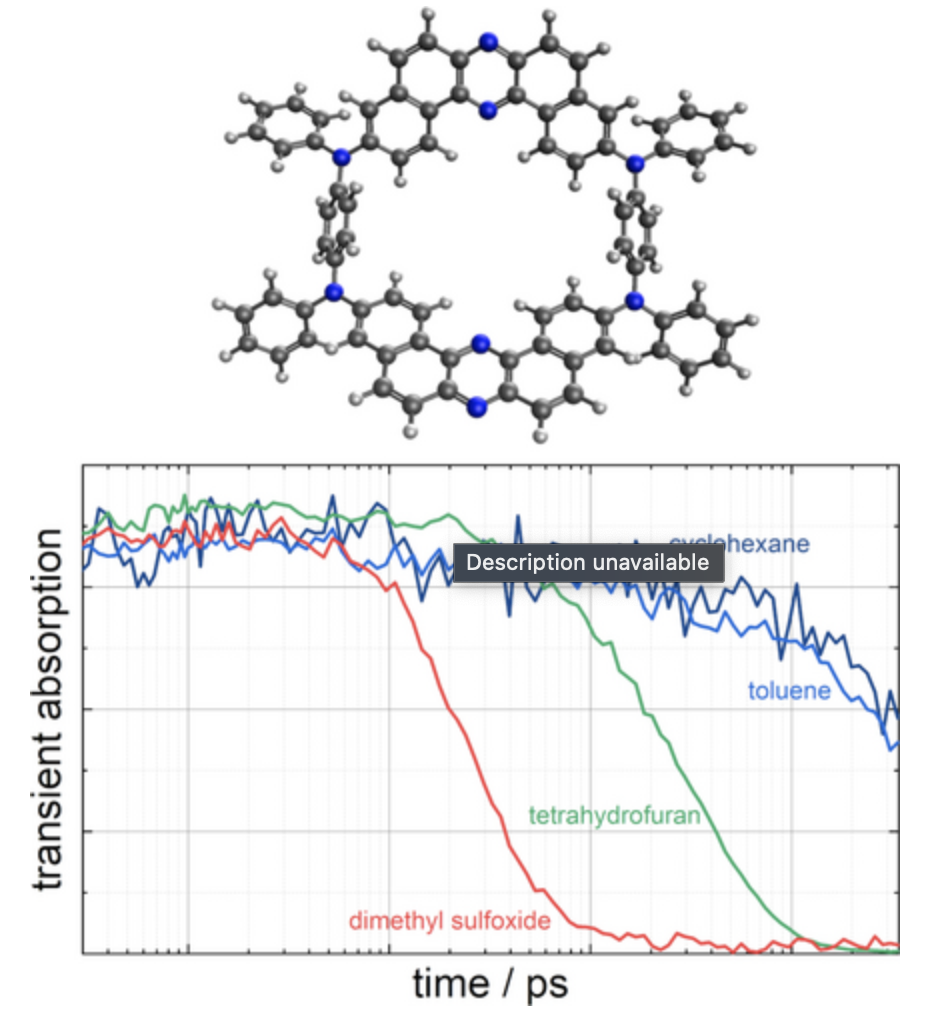
“Tris(pentafluorophenyl)borane-Catalyzed Stereospecific Bromocyanation of Styrene Derivatives with Cyanogen Bromid"
Kensuke Kiyokawa*, Ikumi Noguchi, Takaya Nagata, and Satoshi Minakata*
Org. Lett. 2023, 25, 2537–2542. DOI:10.1021/acs.orglett.3c00727

Abstract: We herein report on the bromocyanation of styrene derivatives with cyanogen bromide in the presence of tris(pentafluorophenyl)borane which functions as a Lewis acid catalyst that can effectively activate cyanogen bromide. This reaction proceeds through a stereospecific syn-addition. The protocol is operationally simple and provides practical access to β-bromonitriles.
“Stereospecific Oxycyanation of Alkenes with Sulfonyl Cyanide"
Kensuke Kiyokawa*, Miu Ishizuka, and Satoshi Minakata*
Angew. Chem. Int. Ed., 2023, 62, e202218743/1–7. DOI:10.1002/anie.202218743
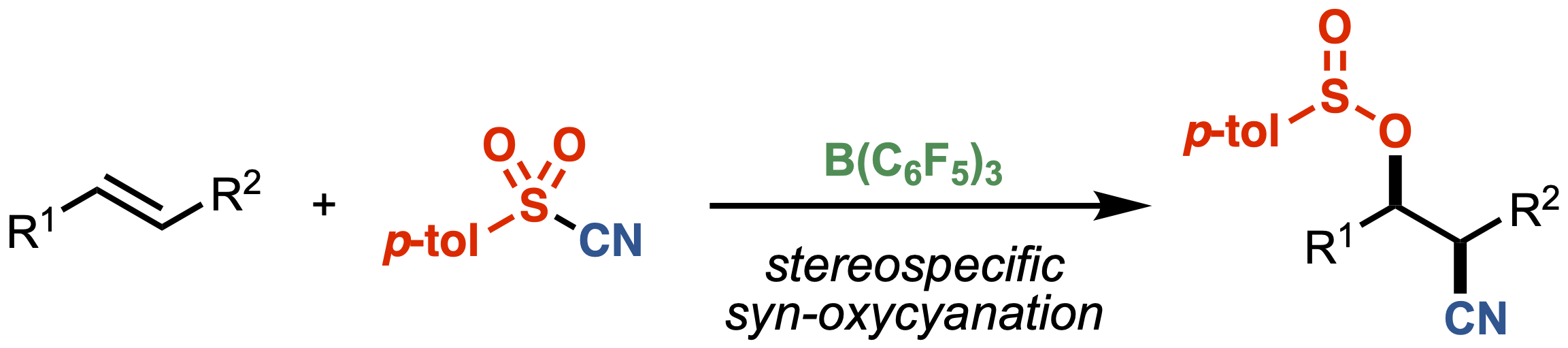
Abstract: Oxycyanation of alkenes would allow the direct construction of useful β-hydroxy nitrile scaffolds. However, only limited examples of such reactions have been reported, and some problems including limited substrate scope and the lack of diastereocontrol in the case of the oxycyanation of internal alkenes have arisen. We herein report on the intermolecular oxycyanation of alkenes using p-toluenesulfonyl cyanide (TsCN) in the presence of tris(pentafluorophenyl)borane (B(C6F5)3) as a catalyst, affording products that contain a sulfinyloxy group and a cyano group at the vicinal position. The reaction features a stereospecific syn-addition. The reaction also shows a broad substrate scope with good functional group tolerance. Mechanistic investigations by experimental studies and density functional theory (DFT) calculations revealed that the reaction proceeds via an unprecedented stereospecific mechanism through the electrophilic cyanation of alkenes, in which B(C6F5)3 efficiently activates TsCN through the coordination of the cyano group to the boron center.
“α-Amination of Carbonyl Compounds by Using Hypervalent Iodine-Based Aminating Reagents Containing a Transferable (Diarylmethylene)amino Group"
Daichi Okumatsu, Kazuki Kawanaka, Shunpei Kainuma, Kensuke Kiyokawa*, and Satoshi Minakata*
Chem. Eur. J., 2023 29, e202203722/1–7. DOI:10.1002/chem.202203722
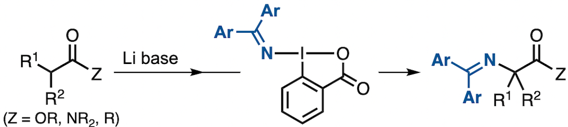
*Selected as the "Cover Feature" of the issue!
Abstract: Hypervalent iodine-based aminating reagents containing a transferable (diarylmethylene)amino group can be used for the α-amination of simple carbonyl compounds such as esters, amides, and ketones in the presence of a lithium base. The (diarylmethylene)amino groups of the products can be readily modified thus providing access to primary amines and diarylmethylamines. The developed method features transition-metal-free conditions and a simple one-pot procedure without the need to prepare enolate equivalents separately, thus offering a general and practical approach to the synthesis of a wide variety of α-amino carbonyl compounds. Experimental mechanistic investigations indicate that this amination proceeds through a unique radical coupling of an α-carbonyl radical with an iminyl radical which are generated through a single-electron transfer between a lithium enolate and the hypervalent iodine reagent.
“3,11-Diaminodibenzo[a,j]phenazine: Synthesis, Properties, and Applications to Tröger's Base-Forming Ladder Polymerization"
Saika Izumi, Keiki Inoue, Yuya Nitta, Tomoya Enjou, Takahiro Ami, Kouki Oka, Norimitsu Tohnai*, Satoshi Minakata, Takanori Fukushima, Fumitaka Ishiwari*, and Youhei Takeda*
Chem. Eur. J., 2023 29, e202202702/1–6. DOI:10.1002/chem.202202702
*Invited as a part of a Special Collection on "Novel Aromatics (ISNA 19)"!
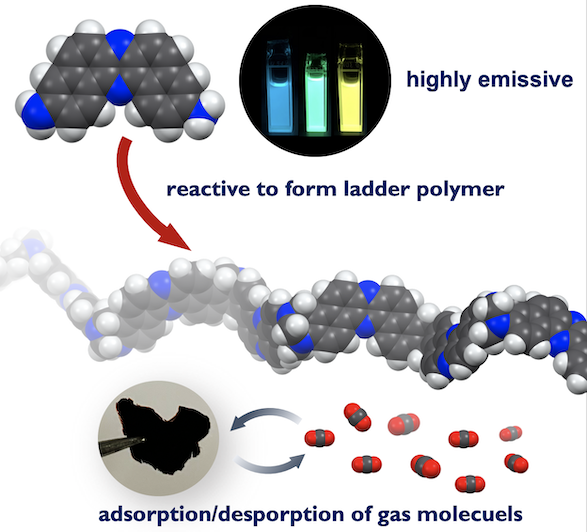
Abstract: A new class of diamino-substituted π-extended phenazine compound was synthesized, and its photophysical properties were investigated. The U-shaped diaminophenazine displayed photoluminescence in solution with moderate quantum yield. The diamino aromatic compound was found applicable to the poly-condensation with formaldehyde to form Tröger’s base ladder polymer. The obtained microporous ladder polymer features high CO2 adsorption selectivity against N2, most likely due to the presence of basic nitrogen atoms in the phenazine rings.