home > Pablication
2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
"Synthesis and Structure of Hypervalent Iodine(III) Reagents Containing Phthalimidate and Application to Oxidative Amination Reactions"
Kensuke Kiyokawa*, Tomoki Kosaka, Takumi Kojima, and Satoshi Minakata*
Angew. Chem., Int. Ed. 2015, 54, 13719–13723. DOI:10.1002/anie.201506805
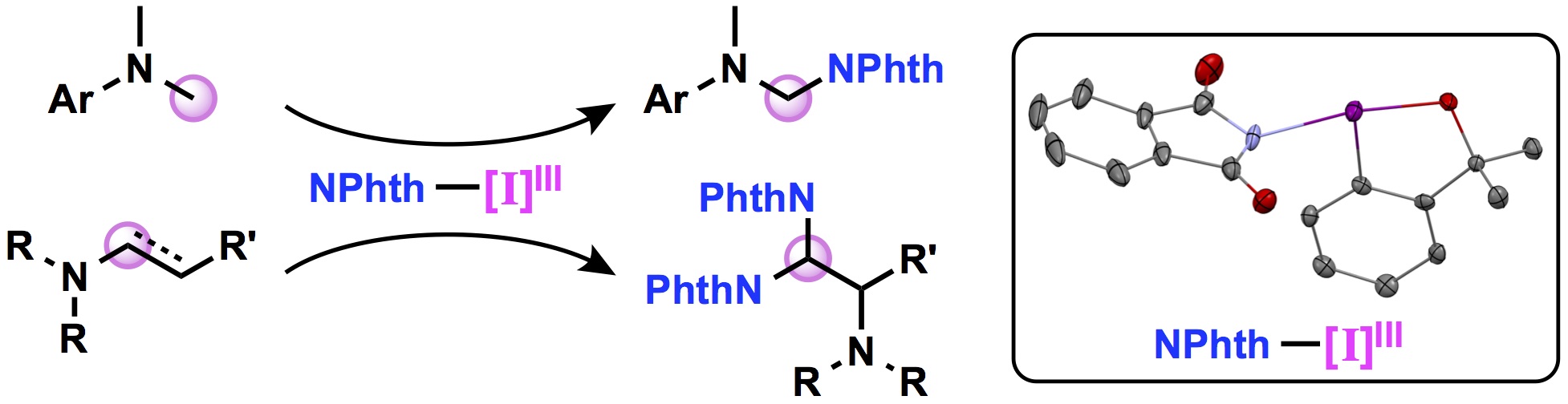
Abstract: A new class of hypervalent iodine reagents containing phthalimidate was synthesized, and structurally characterized by X-ray analysis. The benziodoxole-based reagent displays satisfactory solubility in common organic solvents and is reasonably stable in solution as well as in the solid state. The reagent was used in the oxidative amination of sp3 C–H bond of N,N-dimethylanilines. In addition, the reagent was also applicable to oxidative amination with the rearrangement of trialkylamines as well as enamines that were prepared in situ from secondary amines and aldehydes.
"Iodine-Catalyzed Decarboxylative Amidation of β,γ-Unsaturated Carboxylic Acids with Chloramine Salts Leading to Allylic Amides"
Kensuke Kiyokawa, Takumi Kojima, Yusuke Hishikawa, and Satoshi Minakata*
Chem. Eur. J. 2015, 21, 15548–15552. DOI:10.1002/chem.201503298
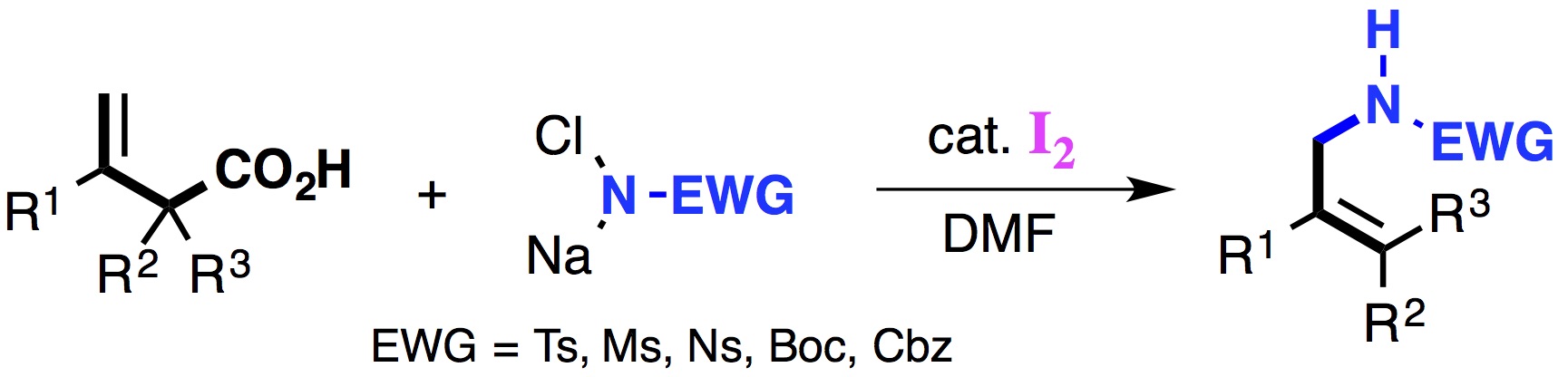
Abstract: The iodine-catalyzed decarboxylative amidation of β,γ-unsaturated carboxylic acids with chloramine salts is desribed. This method enabled the regioselective synthesis of allylic amides from various types of β,γ-unsaturated carboxylic acids containing substituents at the α- and β-positions. In the reaction, N-iodo-N-chloroamides generated by reacting a chloramine salt with I2 function as a key active species. The reaction provides an attractive alternative to existing methods for the synthesis of useful secondary allylic amine derivatives.
"A facile synthesis of functionalized 7,8-diaza[5]helicenes through an oxidative ring-closure of 1,1’-binaphthalene-2,2’-diamines (BINAMs)"
Youhei Takeda*, Masato Okazaki, Yoshiaki Maruoka, and Satoshi Minakata*
Beilstein J. Org. Chem. 2015, 11, 9–15. DOI:10.3762/bjoc.11.2
* Open-access Article!
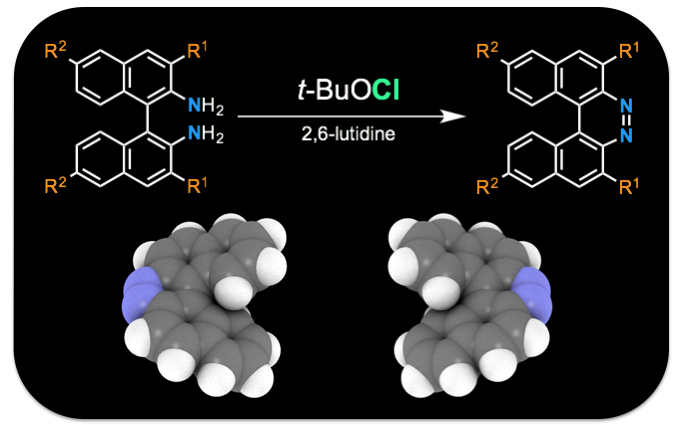
Abstract: A facile and moderately functional-group-tolerant synthetic method for the preparation of 7,8-diaza[5]helicenes has been developed. It comprises of an oxidative ring-closing process of 1,1’-binaphthalene-2,2’-diamine (BINAM) derivatives with a chlorine-containing oxidant (t-BuOCl) in the presence of a base (2,6-lutidine). In addition the basic physicochemical properties of newly synthesized compounds have been investigated.
"2-Halogenoimidazolium Salt Catalyzed Aza-Diels–Alder Reaction through Halogen-Bond Formation"
Youhei Takeda*, Daichi Hisakuni, Chun-Hsuan Lin, and Satoshi Minakata*
Org. Lett. 2015, 17, 318–321. DOI:10.1021/ol503426f
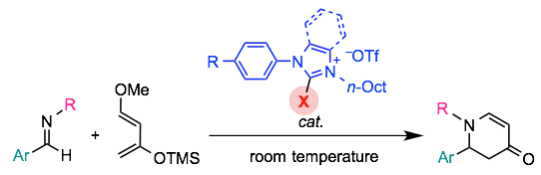
Abstract: 2-Halogenoimidazolium salts are found to catalyze aza-Diels–Alder reaction of aldimines with Danishefsky diene in an efficient manner. Comparative studies and titration experiments support the formation of halogen bonding between imines and catalysts.
"Revisiting Phosphorus Analogues of Phthalimides and Naphthalimides: Syntheses and Comparative Studies"
Youhei Takeda*, Takuya Nishida, Kota Hatanaka, and Satoshi Minakata*
Chem. Eur. J. 2015, 21, 1666–1672. DOI:10.1002/chem.201405494
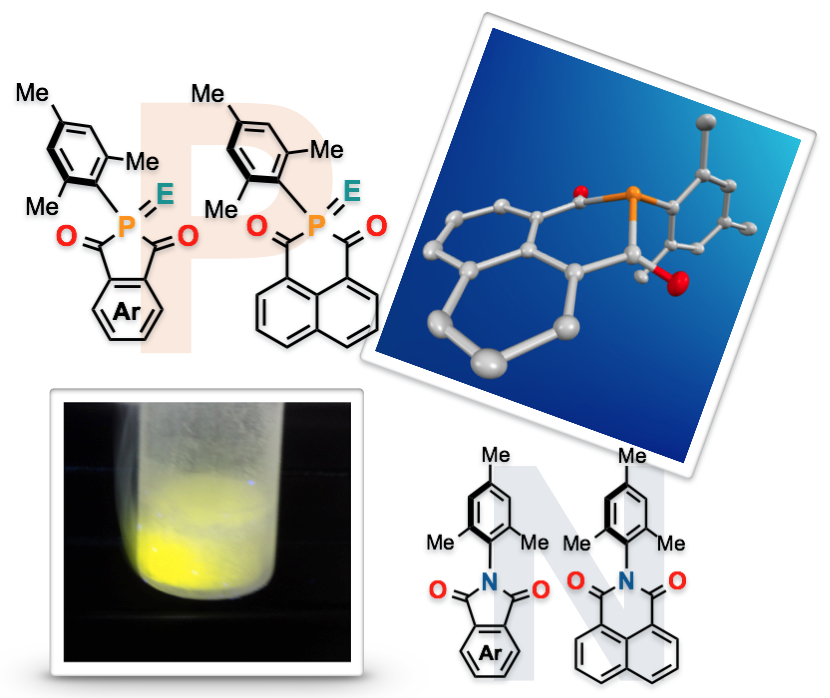
Abstract: A series of phosphorus analogues of aromatic-fused monoimides (phthalimides and naphthalimides) bearing a mesityl group on the P center have been synthesized. In a comparison of their photophysical, electrochemical, and thermal properties with those of the corresponding imides, the impact of P incorporation was revealed. Furthermore, theoretical studies using DFT methods were conducted to understand their properties.
"Straightforward Synthesis of 1,2-Dicyanoalkanes from Nitroalkenes and Silyl Cyanide Mediated by Tetrabutylammonium Fluoride"
Kensuke Kiyokawa, Takaya Nagata, Junpei Hayakawa, and Satoshi Minakata*
Chem. Eur. J. 2015, 21, 1280–1285. DOI:10.1002/chem.201404780
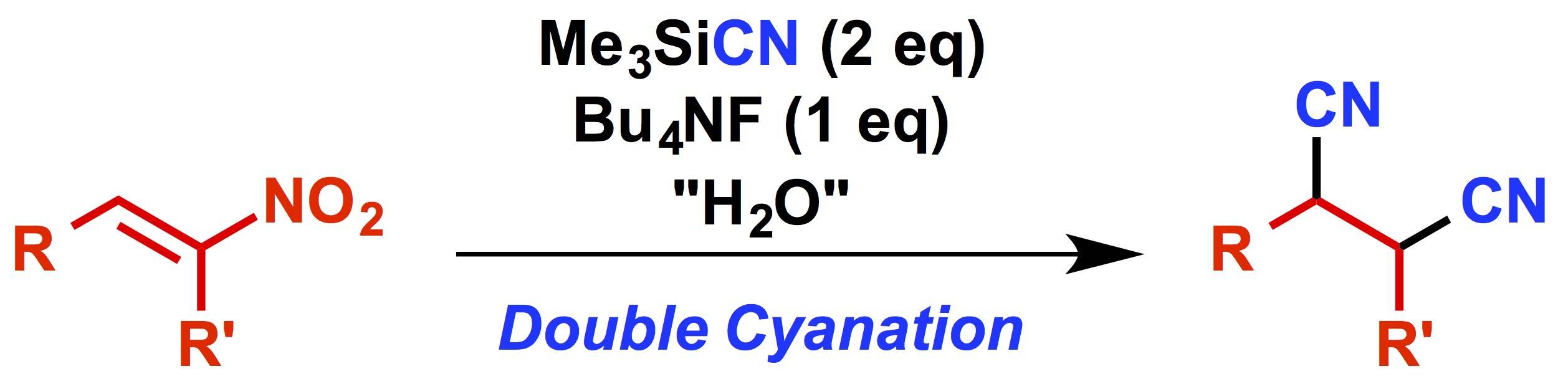
Abstract: A straightforward synthesis of 1,2-dicyanoalkanes by reacting nitroalkenes with trimethylsilyl cyanide in the presence of tetrabutylammonium fluoride is described. The reaction proceeds through a tandem double Michael addition under mild conditions. Employing the hypervalent silicate generated from trimethylsilyl cyanide and tetrabutylammonium fluoride is essential for achieving this transformation. Mechanistic studies suggest that a small amount of water included in the reaction media plays a key role. This protocol is applicable to various types of substrates including electron-rich and electron-deficient aromatic nitroalkenes, and aliphatic nitroalkenes. Moreover, vinyl sulfones were found to be good alternatives, particularly for electron-deficient nitroalkenes. The broad substrate scope and functional group tolerance of the reaction makes this approach a practical method for the synthesis of valuable 1,2-dicyanoalkanes.