home > Pablication
2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
"Hypervalent Iodine(III)-Mediated Oxidative Decarboxylation of β,γ-Unsaturated Carboxylic Acids"
Kensuke Kiyokawa, Shunsuke Yahata, Takumi Kojima, and Satoshi Minakata*
Org. Lett. 2014, 16, 4646–4649. DOI:10.1021/ol5022433
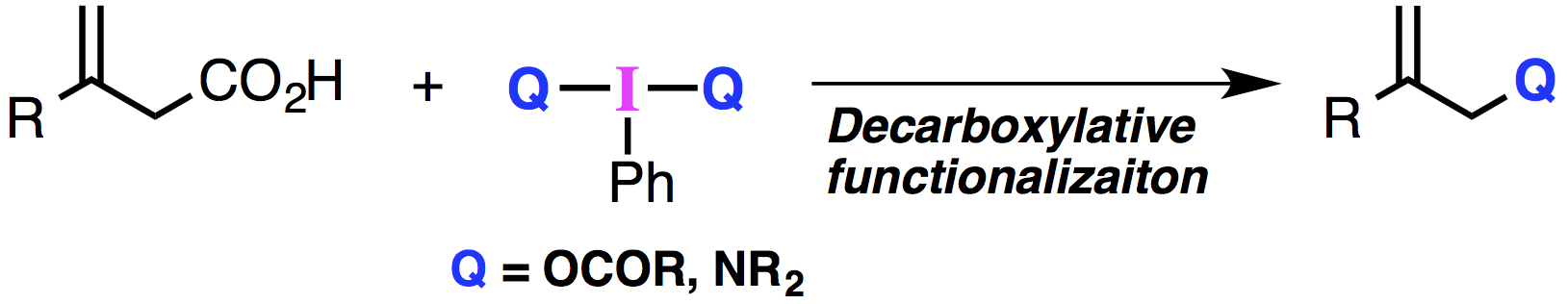
Abstract: A novel oxidative decarboxylation of β,γ-unsaturated carboxylic acids mediated by hypervalent iodine(III) reagents is described. The decarboxylative C–O bond forming reaction proceeded in the presence of PhI(OAc)2 to give the corresponding allylic acetates. In addition, decarboxylative C–N bond formation was achieved by utilizing hypervalent iodine(III) reagents containing an I–N bond. Mechanistic studies suggest the unique reactivity of hypervalent iodine reagents in this ionic oxidative decarboxylation.
"Oxidative Skeletal Rearrangement of 1,1’-Binaphthalene-2,2’-Diamines (BINAMs) via C–C Bond Cleavage and Nitrogen Migration: A Versatile Synthesis of U-Shaped Azaacenes"
Youhei Takeda*, Masato Okazaki, and Satoshi Minakata*
Chem. Commun. 2014, 50, 10291–10294. DOI:10.1039/C4CC04911J
* Open-access Article!
* Highlighted in Synfacts (2014, 10, 1036)!(see the detail)
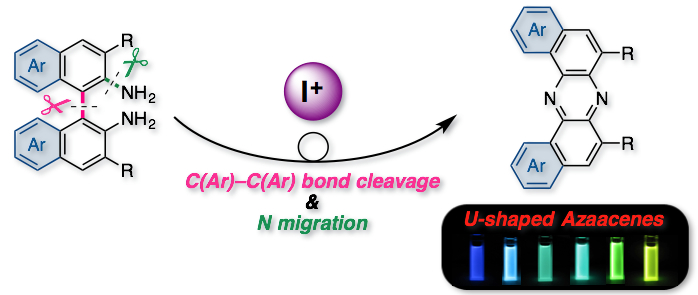
Abstract: An oxidative skeletal rearrangement of 1,1’-binaphthalene-2,2’-diamines (BINAMs) that involves the cleavage of a strong C–C single bond of the binaphthalene unit and the nitrogen migration has been discovered. The unprecedented rearrangement enables access to a series of U-shaped azaacenes otherwise difficult to prepare in a selective manner by classical methods. Moreover, physicochemical properties of the unique azaacenes have been comprehensively investigated.
"2,6-Diphospha-s-indacene-1,3,5,7(2H,6H)-tetraone: A Phosphorus Analogue of Aromatic Diimides with the Minimal Core Exhibiting High Electron-accepting Ability"
Youhei Takeda*, Takuya Nishida, and Satoshi Minakata*
Chem. Eur. J. 2014, 20, 10266–10270. DOI:10.1002/chem.201403735
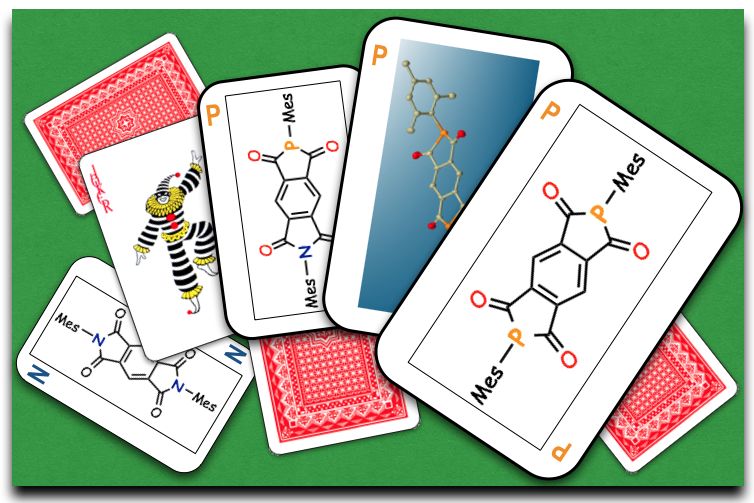
Abstract: Phosphorus analogues of pyrromellitic diimides (PyDIs), which represent a family of privileged electron-accepting organic compounds, have been successfully synthesized as a novel electron-accepting π-conjugated framework. Investigation into their physicochemical properties evidently uncovered their prominent electron-accepting abilities over the corresponding PyDI. Furthermore, theoretical studies revealed the significant contributions of σ*–π* hyperconjugation in the LUMO+1 to stabilize the orbital energies.
"Pd/NHC-Catalyzed Enantiospecific and Regioselective Suzuki-Miyaura Arylation of 2-Arylaziridines: Synthesis of Enantioenriched 2-Arylphenethylamine Derivatives"
Youhei Takeda*, Yuki Ikeda, Akinobu Kuroda, Shino Tanaka, and Satoshi Minakata*
J. Am. Chem. Soc. 2014, 136, 8544–8547. DOI: 10.1021/ja5039616
* Highlighted in Org. Process Res. Dev. as "Some Items of Interest to Process R&D Chemists and Engineers"! !(link)
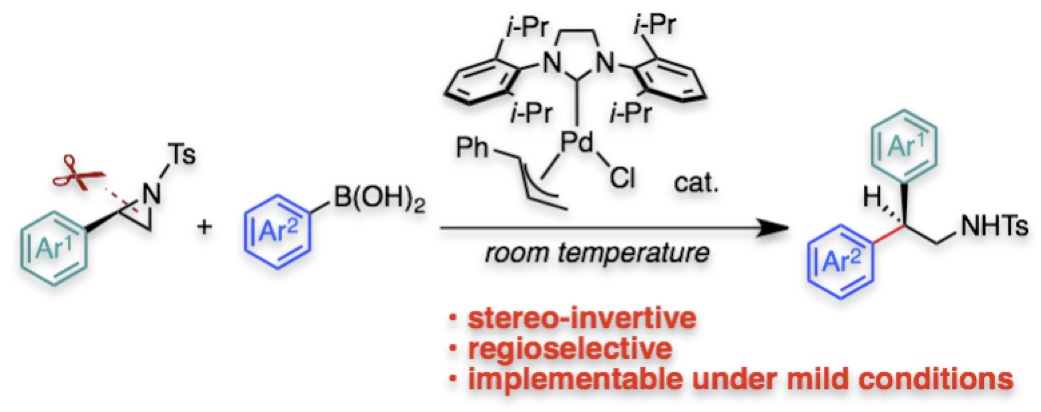
Abstract: A palladium-catalyzed stereospecific and regioselective cross-coupling of enantiopure 2-arylaziridines with arylboronic acids under mild conditions to construct a tertiary stereogenic center has been developed. N-heterocyclic carbene (NHC) ligands drastically promote the coupling, suppressing β-hydride elimination. The enantiospecific cross-coupling allowed us for preparation of a series of biologically important 2-arylphenethylamine derivatives in an enantiopure form.