2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
2015
2014
2013
2012
2011
2010
2009
2008
1998–2007
“Transition-Metal-Free Intramolecular C−H Amination of Sulfamate Esters and N-Alkylsulfamides"
Kensuke Kiyokawa*, Shogo Nakamura, Keisuke Jou, Kohji Iwaida, and Satoshi Minakata*
Chem. Commun. 2019,
55, 11782–11785.
DOI:10.1039/C9CC06410A
*Featured as a part of the themed collection "Editor’s Choice: Main group reagents and catalysts in organic reactions"!
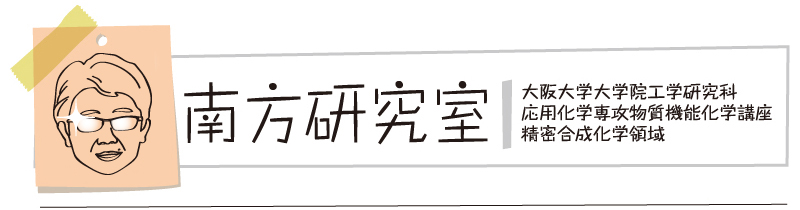
Abstract: The transition-metal-free intramolecular C−H amination of sulfamate esters using iodine oxidants,
tert-butyl hypoiodite (
tBuOI) and
N-iodosuccinimide (NIS) is reported. The reaction proceeds through a 1,6-HAT process via a nitrogen-centered radical, leading to the formation of six-membered oxathiazinane derivatives. A method using NIS was also successfully applied to the oxidative cyclization of
N-alkylsulfamides. The developed protocol, which is operationally simple and proceeds under environmentally benign conditions offers the practical access to synthetically useful 1,3-difunctionalized compounds.
“Electrophilic Amination of Allylic Boranes with Azodicarboxylates: Synthesis of α,α-Disubstituted Allylic Amines"
Kensuke Kiyokawa*, Shunpei Kainuma, and Satoshi Minakata*
Chem. Lett. 2019,
48, 1116–1118.
DOI:10.1246/cl.190448
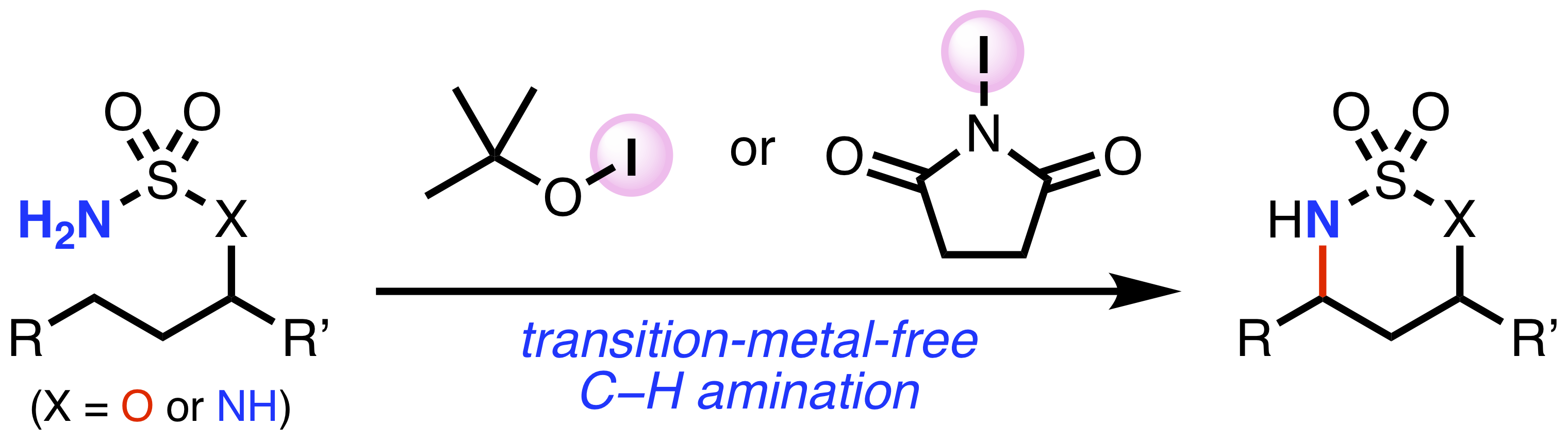
Abstract: The electrophilic amination of allylic boranes, which are prepared from allenes and 9-borabicyclo[3.3.1]nonane (9-BBN), with azodicarboxylates as an aminating reagent is reported. 9-BBN-based allylic boranes were found to be sufficiently reactive to induce an amination reaction, which allows the use of additional activating agents to be avoided. The amination proceeded selectively at the γ-position of the allylic boranes, thus enabling the synthesis of α,α-disubstituted allylic hydrazides by using γ,γ-disubstituted allylic boranes. The products that were synthesized in this amination were readily converted into allylic carbamates. The present method offers a practical route to the production of allylic amine derivatives, which are synthetically useful building blocks.
“Hydrostatic Pressure‐Controlled Ratiometric Luminescence Responses of Dibenzo[a,j]phenazine‐Cored Mechanoluminophore"
Youhei Takeda*, Hiroaki Mizuno, Yusuke Okada, Masato Okazaki, Satoshi Minakata, Thomas Penfold*, and Gaku Fukuhara*
ChemPhotoChem 2019,
3, 1203–1211.
DOI:10.1002/cptc.201900190
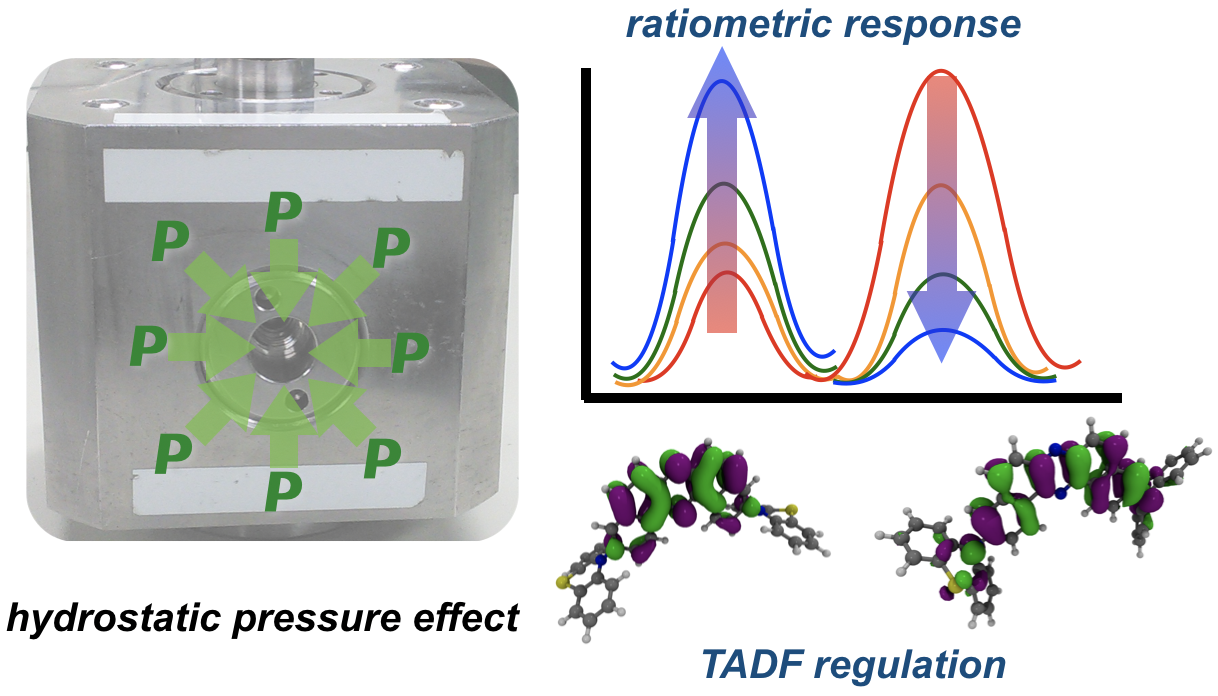
*Selected as the "Front Cover" of the issue!

Abstract: Understanding changes to excited state properties under the influence of an external stimuli, such as pressure or temperature, is important in the context of optimizing molecular components for a number of applications including sensors and imaging reagents. Herein, we use UV/vis, fluorescence and excitation spectroscopies, and fluorescence lifetime measurements supported by calculations to probe the effect of hydrostatic pressure on the excited state characteristics of a conformationally‐divergent mechanochromic compound in toluene and methylcyclohexane. We demonstrate that hydrostatic pressure can be used to manipulate the equilibria between excited state conformers. This work provides new perspectives for mechanoresponsive materials and as attractive alternative to conventional ratiometric sensors.
“Catalyst-Controlled Regiodivergent Ring-Opening C(sp3)–Si Bond-Forming Reactions of 2-Arylaziridines with Silylborane Enabled by Synergistic Palladium/Copper Dual Catalysis"
Youhei Takeda*, Kaoru Shibuta, Shohei Aoki, Norimitsu Tohnai, and Satoshi Minakata*
Chem. Sci. 2019,
10, 8642–8647.
DOI:10.1039/C9SC02507C
*Open-access Article!
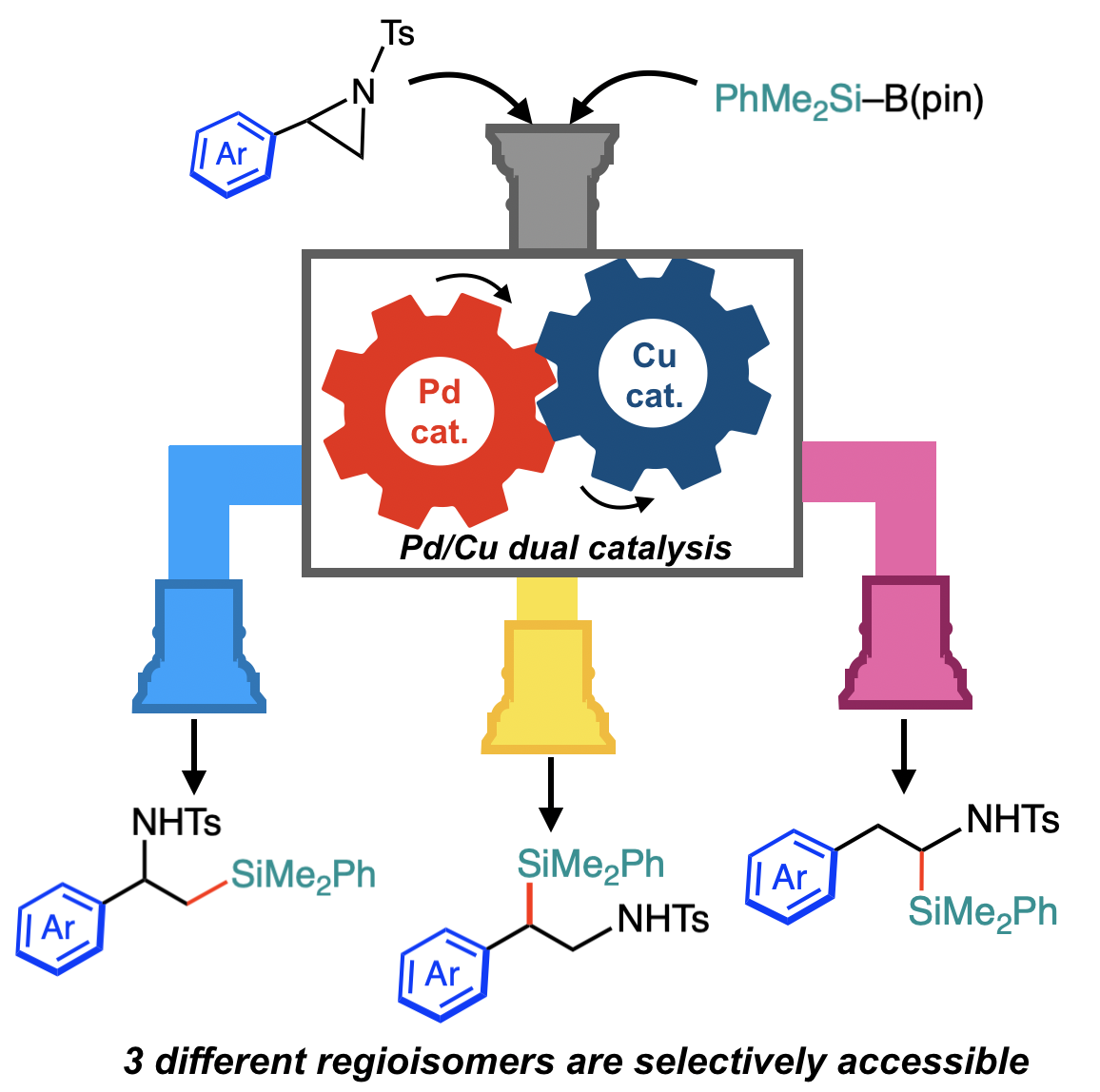
Abstract: A catalyst-controlled regiodivergent and stereospecific ring-opening C(sp
3)–Si cross-couplings of 2-arylaziridines with silylborane enabled by a synergistic Pd/Cu dual catalysis has been developed. Just by selecting suitable combination of catalysts, the regioselectivity of the coupling is completely switched to efficiently provide two regioisomers of β-silylamines (i.e., β-silyl-α-phenethylamines and β-silyl-β-phenethylamines) in good to high yields. Furthermore, a slight modification of the reaction conditions caused a drastic change in reaction pathways, leading to a tandem reaction to produce another regioisomer of silylamine (i.e., α-silyl-β-phenethylamines) in an efficient and selective manner.
“Aromatic-Fused Diketophosphanyl-Core Organic Functional Materials: Phosphorus Mimics of Imides or Beyond?"
Youhei Takeda*, and Satoshi Minakata
Org. Biomol. Chem. 2019,
17, 7807–7821.
DOI:10.1039/C9OB01328H
*Featured as a part of the themed collection "Organic & Biomolecular Chemistry HOT article collection"!
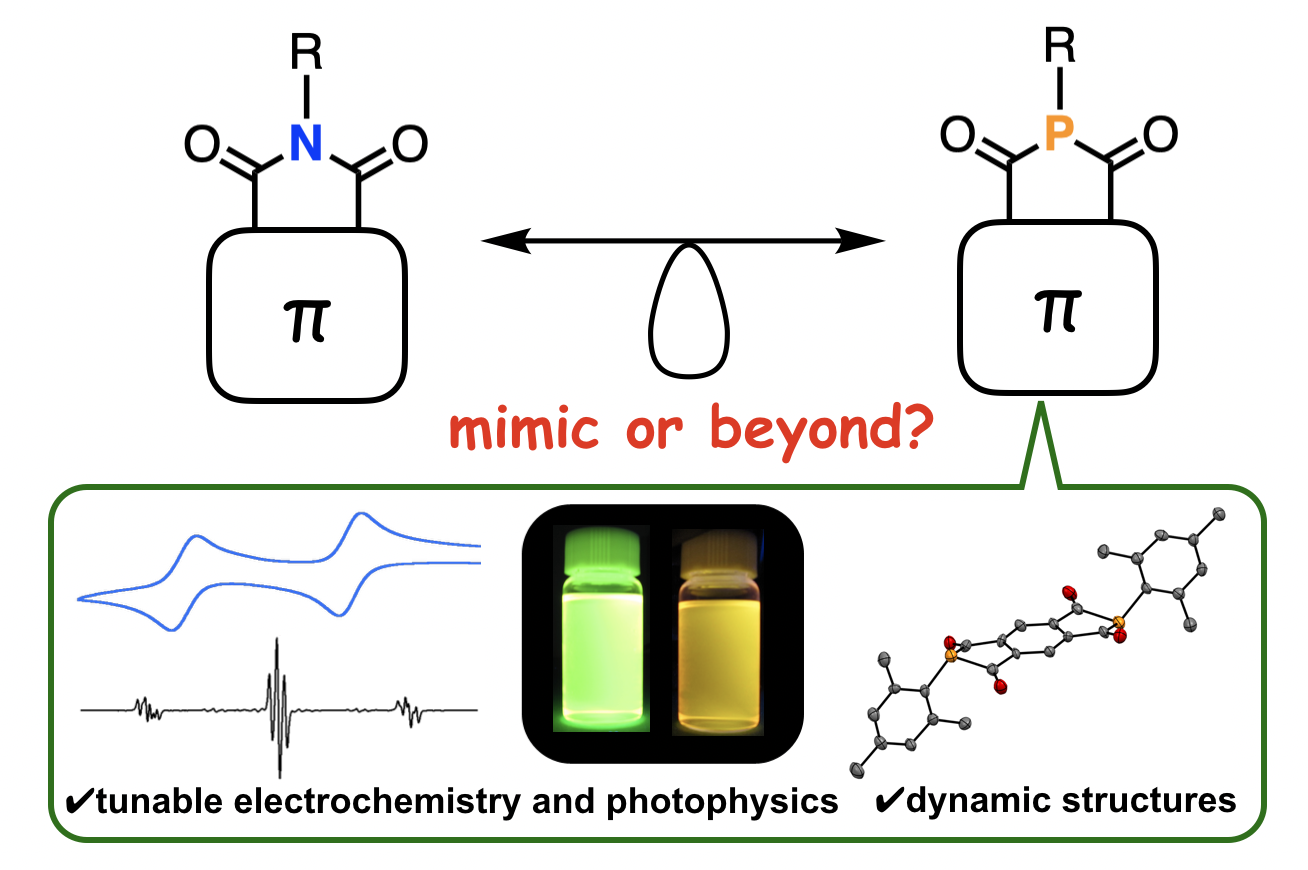
Abstract: Recently, missing pieces of organophosphorus compounds, i.e., aromatic-fused diketophosphanyl compounds, have attracted much attention as promising scaffolds of building blocks for functional organic materials. In this review, the brief historical background, synthetic methods, structures, and optoelectronic aspects of aromatic-fused diketophosphanyls are overviewed.
“Asymmetric Synthesis of β2-Aryl Amino Acids through Pd-Catalyzed Enantiospecific and Regioselective Ring-Opening Suzuki-Miyaura Arylation of Aziridine-2-carboxylates"
Youhei Takeda*, Tetsuya Matsuno, Akhilesh K. Sharma, W. M. C. Sameera*, and Satoshi Minakata*
Chem. Eur. J. 2019,
25, 10226–10231.
DOI:10.1002/chem.20192009
*Highlighted in Synfacts (2019, 15, 1076)! (see the detail)
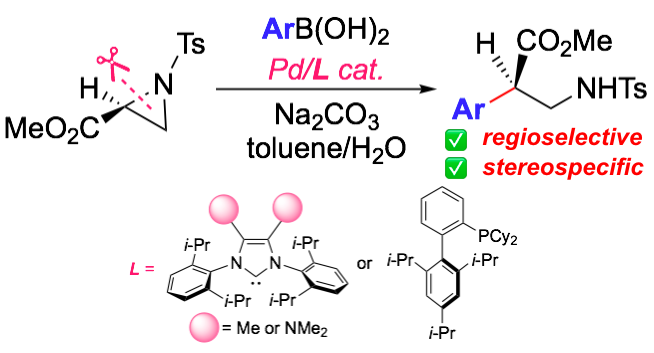
Abstract: A Pd-catalyzed regioselective and enantiospecific ring-opening Suzuki--Miyaura arylation of 2-alkoxycarbonylaziridines was developed. The cross-coupling allowed for the asymmetric preparation of enantiopure β
2-amino acids, starting from commercially available serine esters. Computational studies were conducted to rationalize the results and to propose a plausible catalytic cycle.
“Synthesis of Hypervalent Iodine(III) Reagents Containing a Transferable (Diarylmethylene)amino Group and Their Use in the Oxidative Amination of Silyl Ketene Acetals"
Kensuke Kiyokawa*, Daichi Okumatsu, and Satoshi Minakata*
Angew. Chem. Int. Ed. 2019,
58, 8907–8911.
DOI:10.1002/anie.201904971
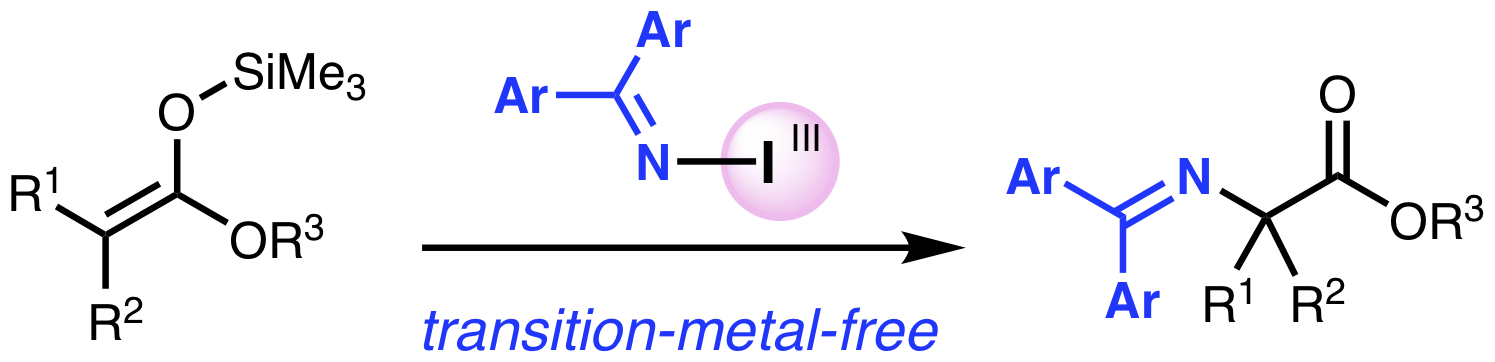
Abstract: The preparation of some hypervalent iodine reagents containing a transferable amino group derived from benzophenone imine derivatives is reported. The reagents can be readily prepared and stored as a bench‐stable solid, and were successfully used in the transition‐metal‐free oxidative amination of silyl ketene acetals to afford the corresponding α‐amino esters, the benzophenone imine moieties of which could be easily hydrolyzed, thereby leading to the formation of primary amines.
“Thermally Activated Delayed Fluorescence vs Room Temperature Phosphorescence by Conformation Control of Organic Single Molecules"
Przemyslaw Data*, Masato Okazaki, Satoshi Minakata, and Youhei Takeda*
J. Mater. Chem. C 2019,
7, 6616–6621.
DOI:10.1039/C9TC00909D
*Invited Article as a part of the themed collection "Functional Organic Materials for Optoelectronic Applications"!
*Selected as a part of the themed collection "2019 Journal of Materials Chemistry C Most Popular Articles"!
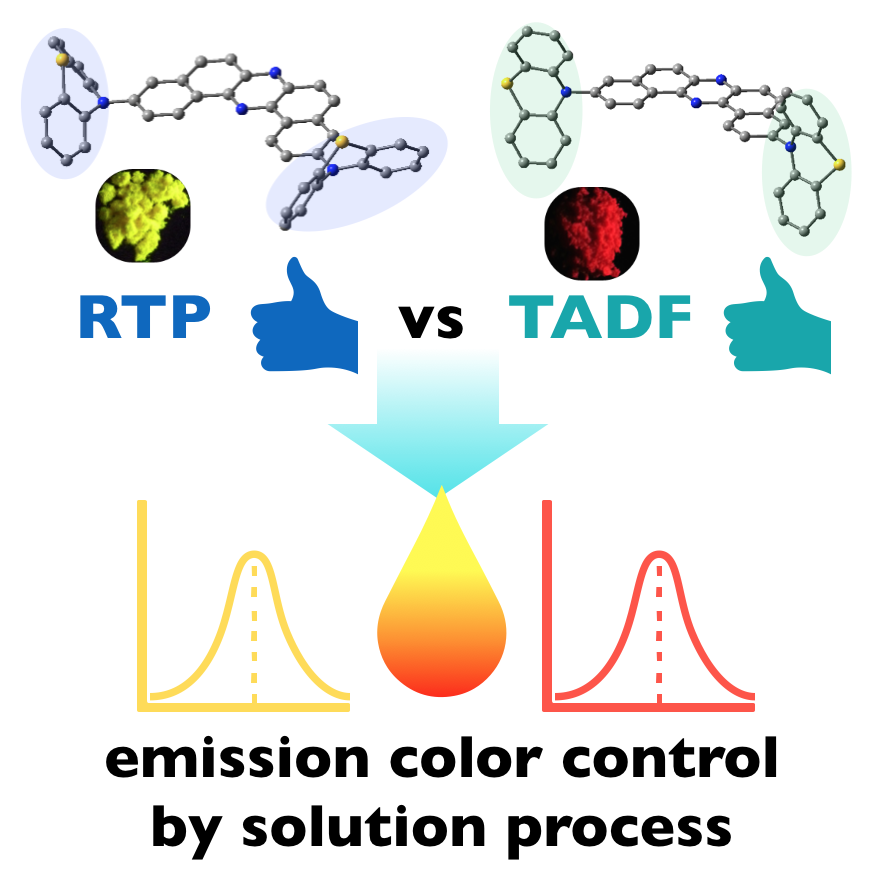
Abstract: The time-resolved photophysial anyalysis of a multi-color-chaning mechanochromic luminescent compound has been disclosed, which reveals distinct different emission paths to boost TADF and RTP of the emitter depending on its molecule conformations. Furthermore, by making the use of the obtained knowledge here, we also present the possibility to control the emission colors by regulating the population of conformers formed inside the emissive layer using solution process, and this technique was applied to fabricate emissive layers of OLED devices.
“A Computational Study on the Mechanism and Origin of the Reigioselectivity and Stereospecificity in Pd/SIPr-Catalyzed Ring-Opening Cross-Coupling of 2-Arylaziridines with Arylboronic Acids"
Akhilesh K. Sharma, W. M. Chamil Sameera*, Youhei Takeda, and Satoshi Minakata
ACS Catal. 2019,
9, 4582–4592.
DOI:10.1021/acscatal.9b01191
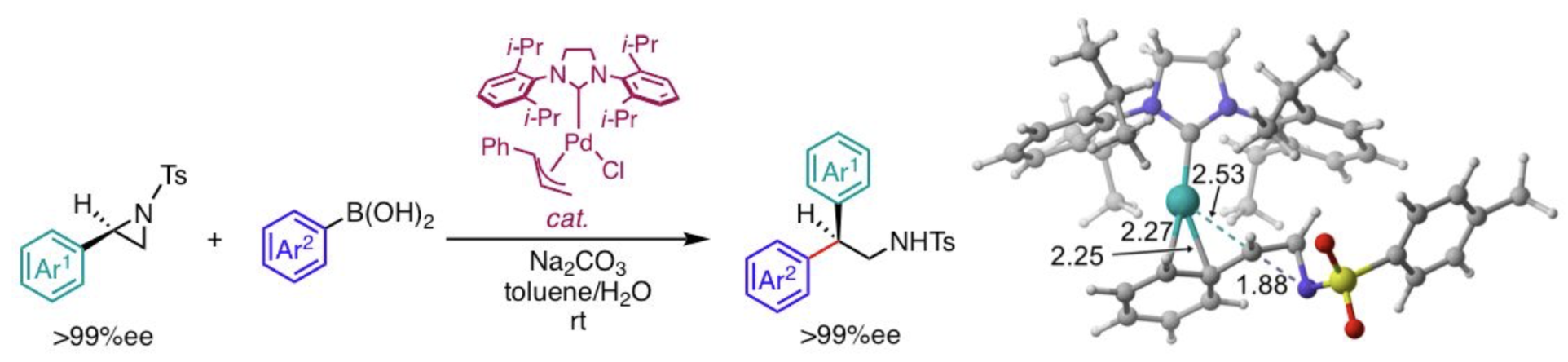
Abstract: The mechanism, regioselectivity, and stereospecificity of Pd/NHC-catalyzed ring-opening cross-coupling of 2-arylaziridines with arylboronic acids (Takeda et al. J. Am. Chem. Soc. 2014, 136, 8544-8547) is rationalized from density functional theory calculations. Pd(0)SIPr complex, the active species, can be formed through the reduction of (
η3-cinnamyl)(Cl)Pd(II)SIPr complex, where arylboronic acid in solution plays a key role. Then, Pd(0)SIPr complex acts as the active species of the catalytic cycle that consists of the regioselective and stereospecific oxidative addition, proton transfer, rate-determining transmetalation, and reductive elimination. Transition states for the oxidative addition were systematically determined from a multi-component artificial force induced reaction (MC-AFIR) search, and explained the regioselectivity and stereospecificity of the reaction. An energy decomposition analysis (EDA) on the key transition states suggested that the interactions between Pd(0)SIPr and 2-arylaziridines are important to the selectivity. Computed mechanism of the full catalytic cycle is consistent with the experimental data. Our detailed mechanistic survey provides important mechanistic insights for enantiospecific and regioselective ring-opening reactions of 2-arylaziridines.
“Ni(II) 10-Phosphacorrole: A Porphyrin Analogue Containing Phosphorus at the Meso Position"
Hiroto Omori, Satoru Hiroto, Youhei Takeda, Heike Fliegl, Satoshi Minakata, and Hiroshi Shinokubo*
J. Am. Chem. Soc. 2019,
141, 4800–4805.
DOI:10.1021/jacs.8b13169
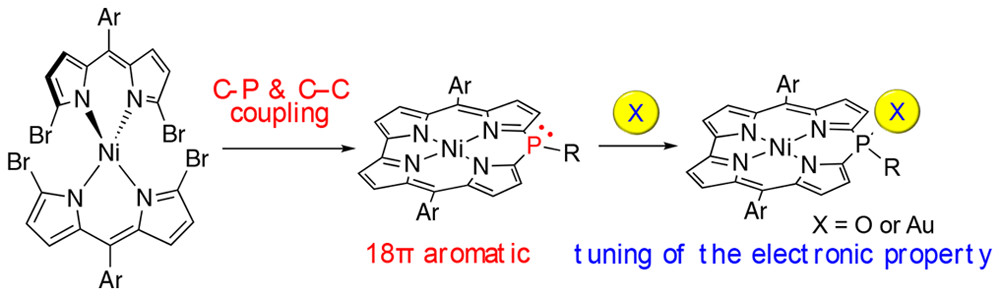
Abstract: Ni(II) 10-Phosphacorrole, a porphyrinoid containing phosphorus at the meso position, was synthesized from a bis(α,α′-dibromodipyrrin) Ni(II) complex and a phosphine anion via the palladium-catalyzed formation of a C–C and two C–P bonds. The optoelectronic properties of Ni(II) 10-phosphacorrole can be modulated effectively by oxidation or coordination of a metal to the phosphorus center. While Ni(II) 10-phosphacorrole exhibits a distinctly aromatic character due to the cyclic conjugation of 18 π-electrons, its oxide exhibited weak antiaromaticity, which was confirmed experimentally and theoretically.
“Electrophilic Cyanation of Allylic Boranes: Synthesis of β,γ-Unsaturated Nitriles Containing Allylic Quaternary Carbon Center"
Kensuke Kiyokawa*, Shotaro Hata, Shumpei Kainuma, and Satoshi Minakata*
Chem. Commun. 2019,
55, 458–461.
DOI:10.1039/C8CC09229J
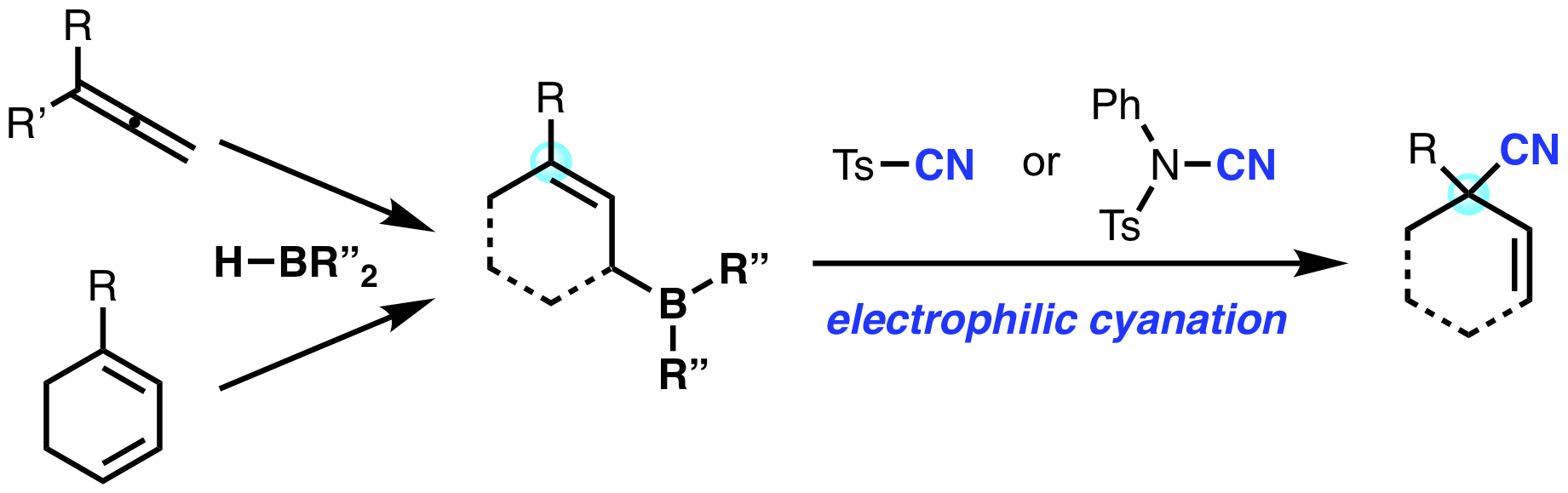
Abstract: The electrophilic cyanation of allylic boranes, a process that is applicable to the construction of allylic quaternary carbon centers, is reported. The reaction has a broad substrate scope with a high functional group tolerance. The results represent an unprecedented and powerful tool for preparing synthetically useful β,γ-unsaturated nitriles, including derivatives that have been difficult to access using existing methods. The synthetic utility of the method was further demonstrated by functional group interconversions of the cyano group of the products.
“The Impact of Replacement of Nitrogen with Phosphorus Atom in the Pyromellitic Diimides on Their Photophysical and Electrochemical Properties"
Sandra Pluczyk*, Heather Higginbotham*, Przemyslaw Data, Youhei Takeda*, and Satoshi Minakata
Electrochim. Acta 2019,
295, 801–809.
DOI:10.1016/j.electacta.2018.10.156
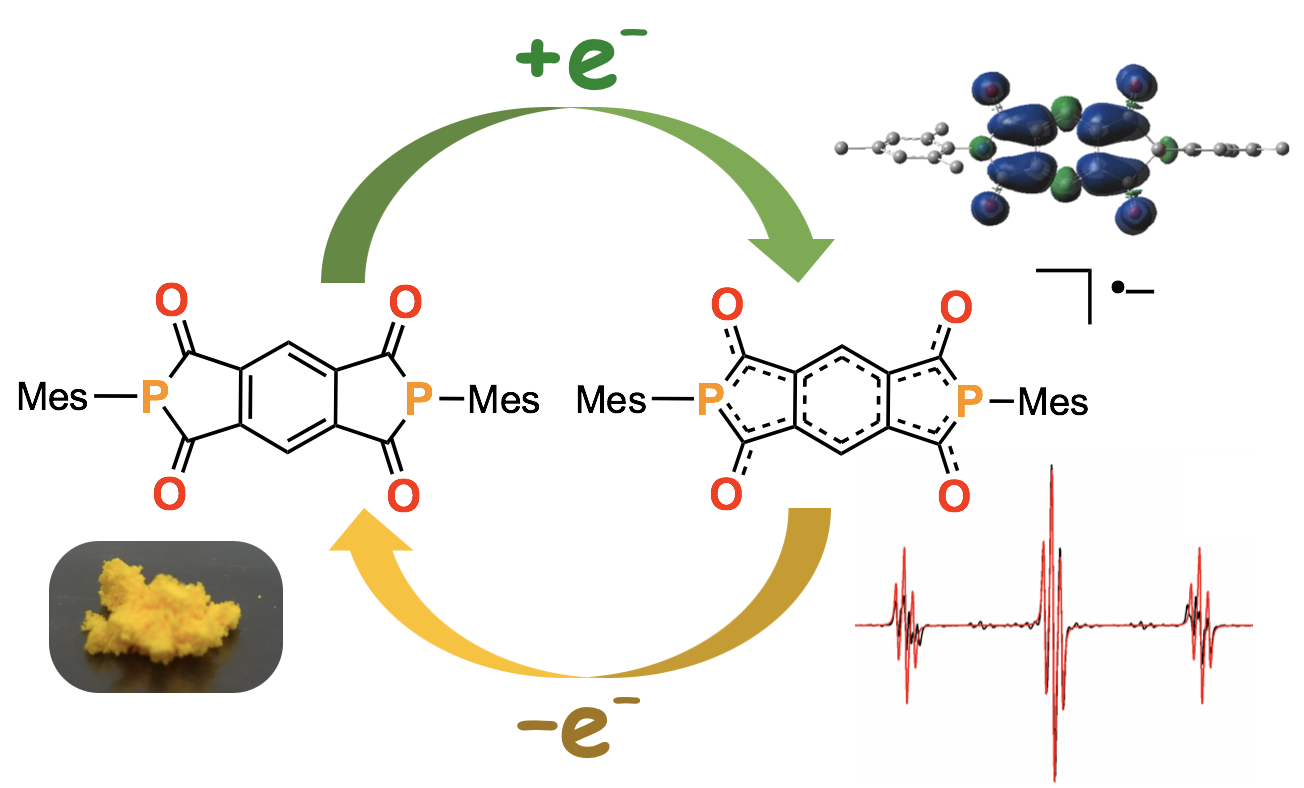
Abstract: Exploration of optoelectronic properties of novel phosphorus-embedded π-conjugated compounds would provide us with fundamental information about the design of hitherto unknown electroactive organic materials. Herein, detailed photophysical and electrochemical profiles of a series of benzene-cored diketophosphanyl compounds were investigated with steady and time-resolved spectroscopic and spectroelectrochemical techniques. The comparative studies revealed the impact of phosphorus and nitrogen atoms on their triplet energies and on the behaviour of electrochemical processes to form radical species.