2017 2016 2015 2014 2013 2012 2011 2010
2009 2008 1998–2007
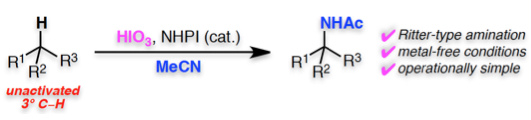
"Ritter-Type Amination of C–H Bonds at Tertiary Carbon Centers Using Iodic Acid as an Oxidant"
Kensuke Kiyokawa*, Kenta Takemoto, and Satoshi Minakata*
Chem. Commun. 2016, 52, 13082–13085. DOI:10.1039/C6CC07164C
*Open-access Article!
*Selected as the "Front Cover" of the issue (Issue 89, 2016)!
Abstract: The Ritter-type amination of a tertiary C–H bond using iodic acid (HIO3) as an oxidant, in the presence of N-hydroxyphthalimide (NHPI) is reported. This operationally simple method is conducted under metal-free conditions, has a wide substrate scope, is scalable, and efficiently provides valuable α-tertiary amine derivatives. Mechanistic investigations suggest that the reaction proceeds via a unique reaction pathway involving the formation of an alkyl iodide and a higher oxidation state iodine(III) species as an intermediate that subsequently undergoes a Ritter-type amination.
"Electrophilic Cyanation of Boron Enolates: Efficient Access to Various β-Ketonitrile Derivatives"
Kensuke Kiyokawa*, Takaya Nagata, and Satoshi Minakata*
Angew. Chem., Int. Ed. 2016, 55, 10458–10462. DOI:10.1002/anie.201605445
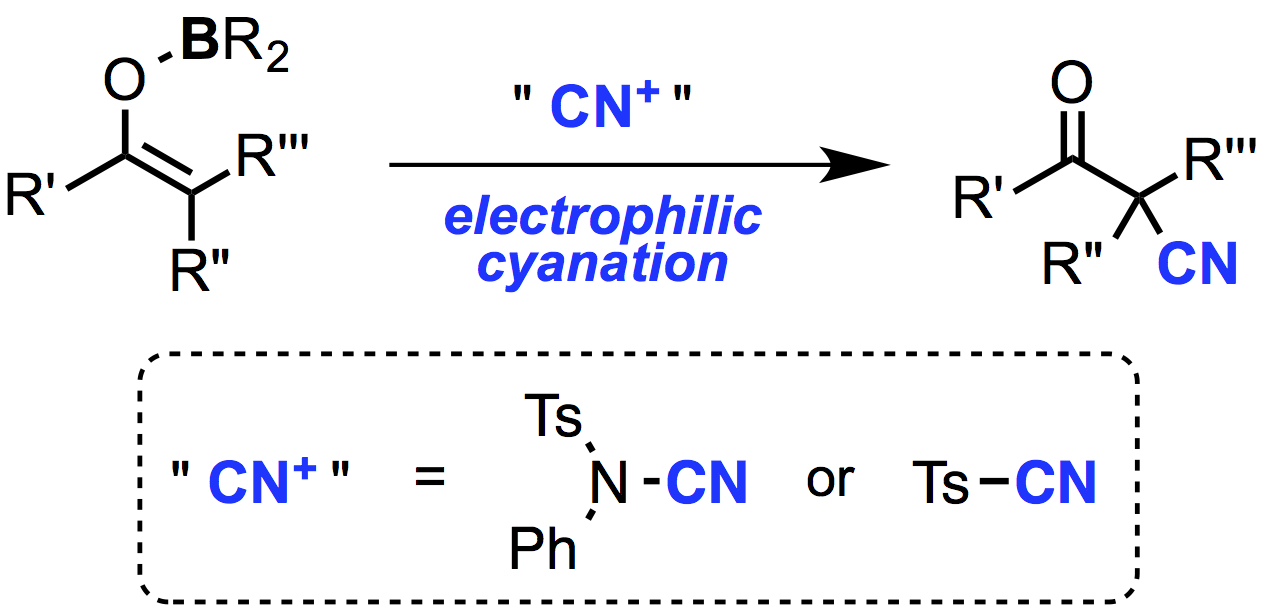
Abstract: The highly efficient electrophilic cyanation of boron enolates using readily available cyanating reagents, N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) and p-toluenesulfonyl cyanide (TsCN), is reported. Various β-ketonitriles were prepared by this new protocol, which has a remarkably broad substrate scope compared to existing methods. The present method also allowed efficient synthesis of β-ketonitriles containing a quaternary α-carbon center.
"Palladium-Catalyzed Regioselective and Stereo-invertive Ring-Opening Borylation of 2-Arylaziridines with Bis(pinacolato)diboron: Experimental and Computational Studies"
Youhei Takeda*, Akinobu Kuroda, W. M. C. Sameera, Keiji Morokuma*, and Satoshi Minakata*
Chem. Sci. 2016, 7, 6141–6152. DOI:10.1039/C6SC01120A
*Open-access Article!
*Highlighted in J. Synth. Org. Chem. Jpn.!
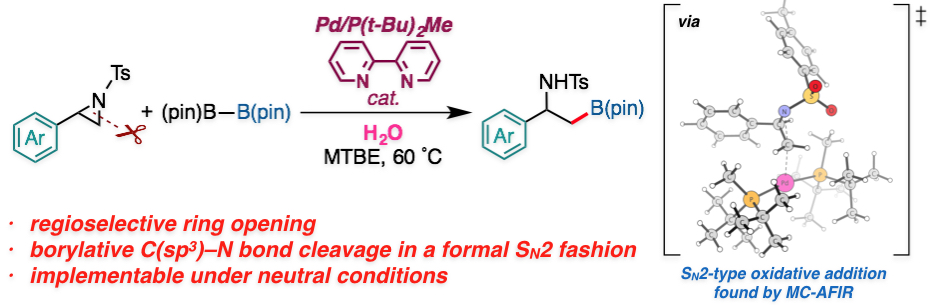
Abstract: A palladium-catalyzed regioselective borylative ring-opening reaction of 2-arylaziridines to give β-amino-β-arylethylborates was developed. The reaction reported herein represents the first example of ring-opening borylation of nonvinylic aziridines and direct borylative C(sp3)–N bond cleavage of neutral organic substrates. NMR studies and density functional theory (DFT) calculations suggested that the active intermediate for the reaction is PdL2 complex [L = P(t-Bu)2Me]. The multi-component artificial force-induced reaction method (MC-AFIR) located the transition states for the regioselectivity-determining aziridine ring opening that proceeds in an SN2 fashion, and explained the selectivity of the reaction. The full catalytic cycle consists of the selectivity-determining aziridine ring opening (oxidative addition), a proton transfer, phosphine ligand dissociation from the catalyst, boron–boron bond cleavage, and reductive elimination. Water is important to the drive the transmetalation step. The calculated overall mechanism and selectivity are consistent with the experimental results.
"Thieno[3,4-c]phosphole-4,6-dione: A Versatile Building Block for Phosphorus-containing Functional π-Conjugated Systems"
Youhei Takeda*, Kota Hatanaka, Takuya Nishida, and Satoshi Minakata*
Chem. Eur. J. 2016, 22, 10360–10364. DOI:10.1002/chem.201602392
*Highlighted in Atlas of Science!
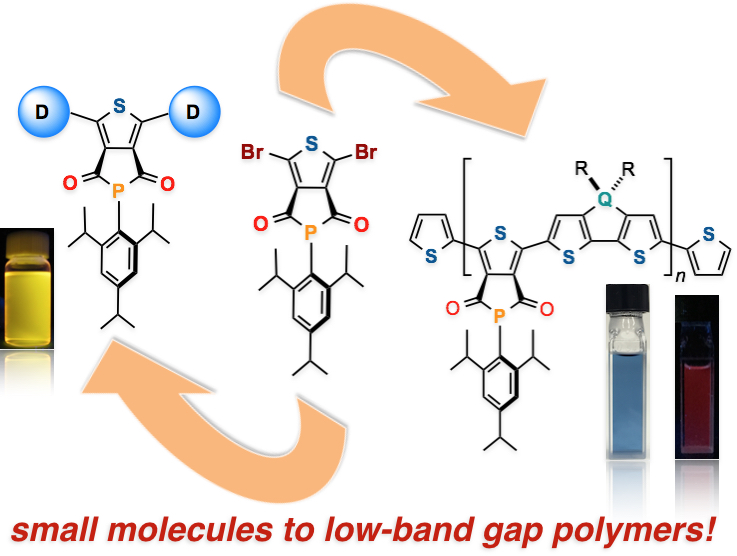
Abstract: A novel and versatile phosphorus-containing π-conjugated building block, thieno[3,4-c]phosphole-4,6-dione (TPHODO), has been developed. The utility of this simple but hitherto unknown building block has been demonstrated by preparing novel functional organophosphorus compounds and bandgap-tunable conjugated polymers.
"Dibenzo[a,j]phenazine-Cored Donor-Acceptor-Donor Compounds as Green-to-Red/NIR Thermally Activated Delayed Fluorescence Organic Light Emitters"
Przemyslaw Data*, Piotr Pander, Masato Okazaki, Youhei Takeda*, Satoshi Minakata, and Andrew P. Monkman
Angew. Chem., Int. Ed. 2016, 55, 5739–5744. DOI:10.1002/anie.201600113
*Highlighted in ResOU, AlphaGalileo, EurekAlert!, ScienceDaily, Materials Today, Phys Org, and Optronics!
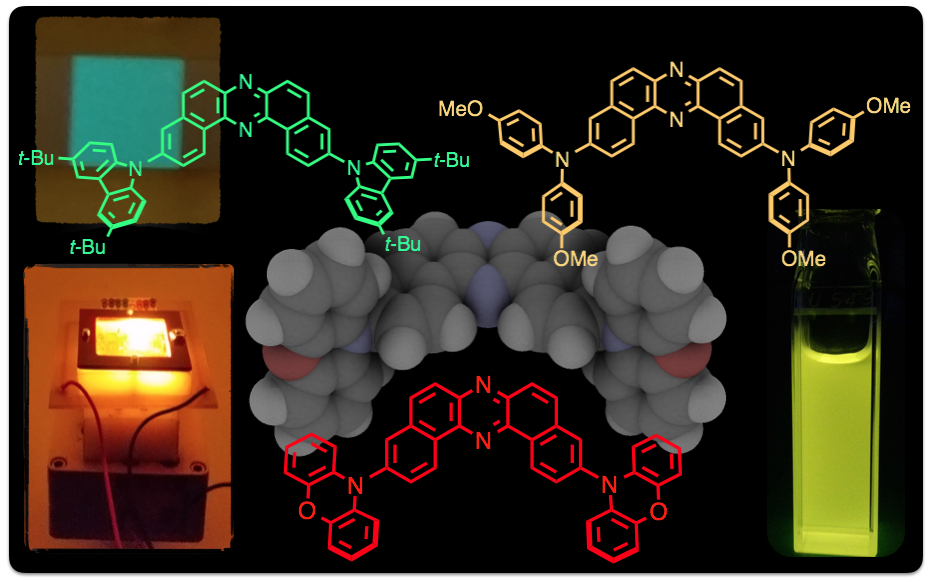
Abstract: A new family of thermally activated delayed fluorescence (TADF) emitters based on U-shaped D-A-D architecture with a novel accepting unit has been developed. All investigated compounds have small singlet-triplet energy splitting (ΔEST) ranging from 0.02 to 0.20 eV and showed efficient TADF properties. The lowest triplet state of the acceptor unit plays the key role in the TADF mechanism. OLEDs fabricated with these TADF emitters achieved excellent efficiencies up to 16 % external quantum efficiency (EQE).
"Ring-contractive and -Closing Skeletal Rearrangement of 1,1’-Binaphthalene-2,2’-Diamines (Binams) Induced by An Iodine-Containing Oxidant: Synthesis of Spiro[Benzo[e]Indole-1,1'- inden]-2-Amines and Application to An Aiee-active Bf2 Complex"
Masato Okazaki, Kosuke Takahashi, Youhei Takeda*, and Satoshi Minakata*
Heterocycles 2016, 93, 770–782. (a Special Issue in honor of Professor Dr. Lutz F. Tietze on 75th Birthday) DOI:10.3987/COM-15-S(T)33
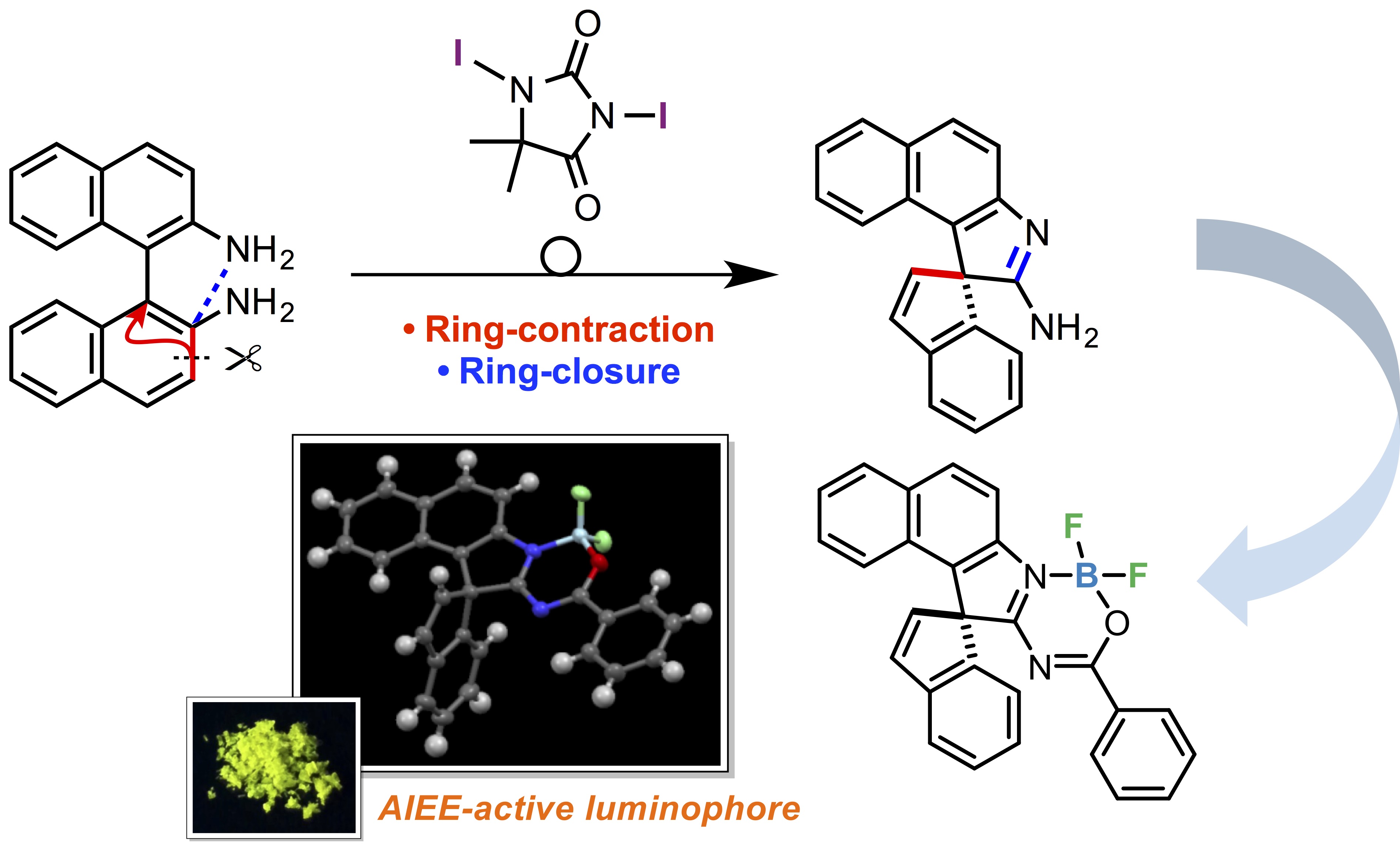
Abstract: An iodine-containing oxidant-induced ring-contractive and -closing skeletal rearrangement of 1,1’-binaphthalene-2,2’-diamines (BINAMs) to afford spiro[benzo[e]indole-1,1'-inden]-2-amines has been discovered. Furthermore, a spiro product was successfully transformed into a novel luminescent spirocyclic BF2 complex.