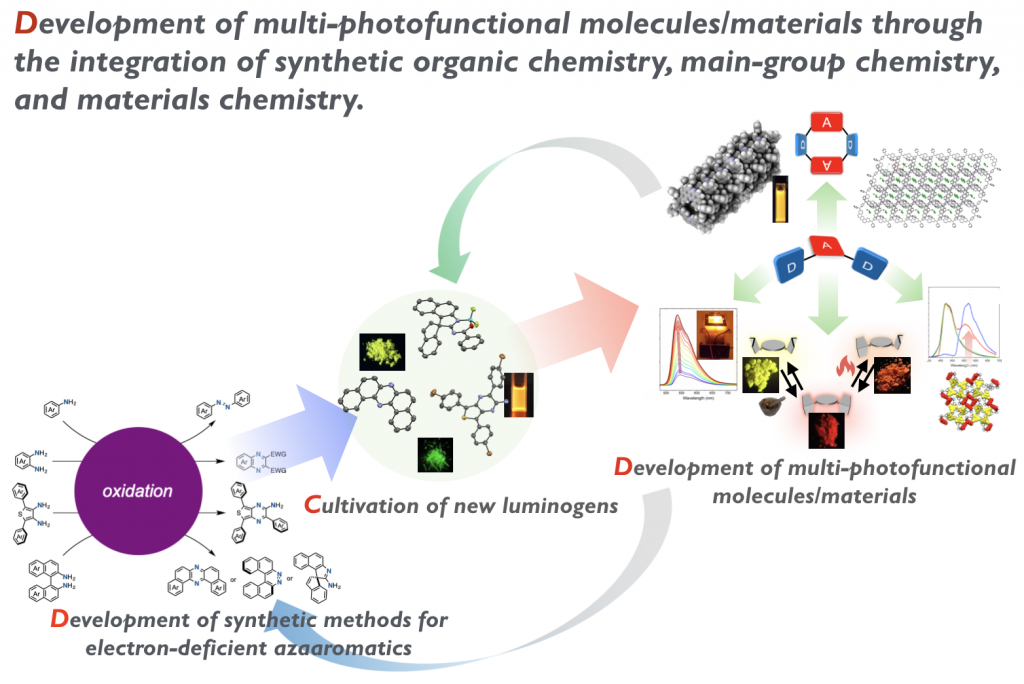
Organic molecules have infinite potentials as functional materials, due to the huge diversity in chemical structures and molecular conformations, electronic configurations, and assembling modes through supramolecular interactions. Nevertheless, the chemical space of organic photofunctional molecules has significantly remained to be explored much more and more. It is highly important to find out ground-breaking design principles for organic photofunctional materials that can give us solutions for existing issues in optoelectronic applications or can pave new avenues toward totally-unexpected arenas.
Over the last years, we have explored a new chemical space of organic molecules through the integrated processes comprising of reaction development and investigation of structure-photophysical property relationship (SPPR). As the results, our team has showcased that twisting electron-donor–acceptor-donor (D-A-D) compounds centered with a uniquely U-shaped azaaromatic serve as a promising scaffold for multi-photofunctional organic materials exhbiting thermally activated delayed fluorescence (TADF), mechanochromic luminescence (MCL), and room-temperature phosphorescence (RTP). What is more, some of them serve as organic emitters for efficient organic light-emitting diodes (OLEDs) and the external quantum efficiencies (EQEs) of the devices far exceeds the theoretical values (ca. 5%) of the OLEDs with the 1st generation fluorescence emitters. Through the series of research, we have drawn an important conclusion that one of the key design elements for developing multi-photofunctional materials with a single molecular structure is the diversity of molecular conformations. Currently, we are exploring a new dimension of chemical space by making full use of our knowledge on the design of multiphotofunctional molecules to discover new ground-breaking materials.
Selected Publication
“Anion-Responsive Colorimetric and Fluorometric Red-Shift in Triarylborane Derivatives: Dual Role of Phenazaborine as Lewis Acid and Electron Donor”
Aota, N.; Nakagawa, R.; de Sousa, L. E.; Tohnai, N.; Minakata, S.; de Silva, P.*; Takeda, Y.*
Angewandte Chemie International Edition 2024, Early View, e202405158/1–10.
[DOI:10.1002/anie.202405158]
“Heavy-Atom-Free Room-Temperature Phosphorescent Organic Light-Emitting Diodes Enabled by Excited States Engineering”
Higginbotham, H. F.; Okazaki, M.; de Silva, P.*; Minakata, S.; Takeda, Y.*; Data, P.*
ACS Appl. Mater. Interfaces 2021, 13, 2899–2907. [DOI: 10.1021/acsami.0c17295]
“Thermally Activated Delayed Fluorescent Donor–Acceptor–Donor–Acceptor π-Conjugated Macrocycle for Organic Light-Emitting Diodes”
Izumi, S.; Higginbotham, H. F.; Nyga, A.; Stachelek, P.; Tohnai, N.; de Silva, P.; Data, P.*; Takeda, Y.*; Minakata, S.*
J. Am. Chem. Soc. 2020, 142, 1482–1491. [DOI: 10.1021/jacs.9b11578]
“Conformationally-Flexible and Moderately Electron-Donating Units-Installed D-A-D Triad Enabling Multi-Color-Changing Mechanochromic Luminescence, TADF and Room-Temperature Phosphorescence”
Takeda, Y.*; Kaihara, T.; Okazaki, M.; Higginbotham, H.; Data, P.*; Tohnai, N.; Minakata, S.
Chem. Commun. 2018, 54, 6847–6850. [DOI: 10.1039/c8cc02365d]
“Thermally Activated Delayed Fluorescent Phenothiazine-dibenzo[a,j]phenazine-phenothiazine Triads Exhibiting Tricolor-Changing Mechanochromic Luminescence”
Okazaki, M.; Takeda, Y.*; Data, P.*; Pander, P.; Higginbotham, H.; Monkman, A. P.; Minakata, S.*
Chem. Sci. 2017, 8, 2677–2686. [DOI: 10.1039/C6SC04863C]
“Dibenzo[a,j]phenzine-Cored Donor-Acceptor-Donor (D-A-D) Compounds as Green-to-Red/NIR Thermally Activated Delayed Fluorescence Organic Light Emitters”
Data, P.*; Pander, P.; Okazaki, M.; Takeda, Y.*; Minakata, S.; Monkman, A. P.
Angew. Chem., Int. Ed. 2016, 55, 5739–5744. [DOI: 10.1002/anie.201600113]
“Oxidative Skeletal Rearrangement of 1,1’-Binaphthalene-2,2’-Diamines (BINAMs) via C–C Bond Cleavage and Nitrogen Migration: A Versatile Synthesis of U-Shaped Azaacenes”
Takeda, Y.*; Okazaki, M.; Minakata, S.*
Chem. Commun. 2014, 50, 10291–10294. [DOI: 10.1039/C4CC04911J]
If you want read more… visit the Publications page.


