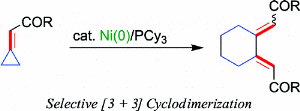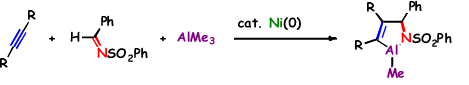Publication
Intramolecular Oxidative Cyclization of Alkenes and Nitriles with Nickel(0)
M. Ohashi, M. Ikawa, and S. Ogoshi
Organometallics, Article ASAP DOI: 10.1021/om2001603
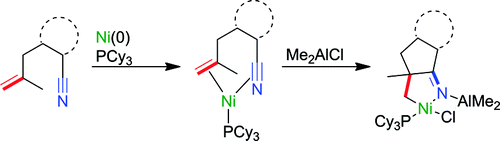
The use of Me2AlCl as an additive was found to allow the oxidative addition of the Ar–CN bond in 2-(2-methylallyl)benzonitrile on nickel(0) in the presence of PnBu3, giving a trans-arylnickelcyanide complex. In contrast, in the presence of PCy3, the intramolecular oxidative cyclization on nickel(0) took place to afford a nickeladihydropyrrole. Without the addition of Me2AlCl, the quantitative generation of an η2:η2-5-ene-nitrile Ni(0) species, which was definitely converted to the nickeladihydropyrrole after treatment with Me2AlCl, was observed.
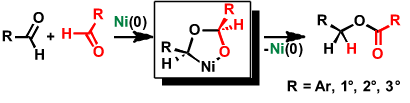
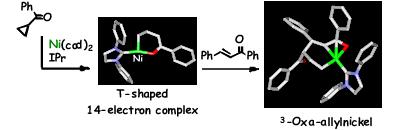
Nickel-Catalyzed Selective Conversion of Two Different Aldehydes to Cross-Coupled Esters
Y. Hoshimoto, M. Ohashi, and S. Ogoshi
J. Am. Chem. Soc., 2011, 133, 4668-4671.
1st place in Most downloaded articles for the previous month!!
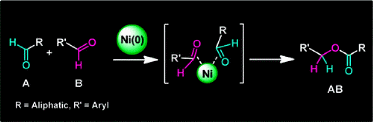
In the presence of a Ni(0)/NHC catalyst, an equimolar mixture of aliphatic and aryl aldehydes can be employed to selectively yield a single cross-coupled ester. This reaction can be applied to a variety of aliphatic (1°, 2°, cyc-2°, and 3°) and aryl aldehyde combinations. The reaction represents 100% atom efficiency and generates no waste. Mechanistic studies have revealed that the striking feature of the reaction is the simultaneous coordination of two aldehydes to Ni(0).
Palladium-catalyzed coupling reactions of tetrafluoroethylene with arylzinc compounds
M. Ohashi, T. Kambara, T. Hatanaka, H. Saijo, R. Doi, and S. Ogoshi
J. Am. Chem. Soc., 2011, 133, 3256-3259.
Selected as Most downloaded articles for the previous month!!
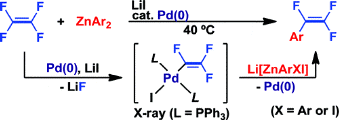
The first Pd-catalyzed monoarylation of tetrafluoroethylene (TFE) with in situ-prepared diarylzinc reagents yielded α,β,β-trifluorostyrene derivatives in excellent yields with high selectivity. C-F bond activation of TFE was achieved by the synergetic effects of the Pd(0) species and LiI and generated a trifluorovinyl palladium(II) intermediate.
Nickel(0)-Catalyzed Formation of Oxaaluminacyclopentenes via an Oxanickelacyclopentene Key Intermediate: Me2AlOTf-Assisted Oxidative Cyclization of an Aldehyde and an Alkyne with Nickel(0)
M. Ohashi, H. Saijo, T. Arai, and S. Ogoshi
Organometallics 2010, 29, 6534-6540.
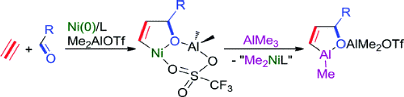
Oxidative cyclization of aldehydes and alkynes with Ni(0) was found to be promoted by addition of Me2AlOTf to yield oxanickelacyclopentenes, which reacted with AlMe3 to be converted the corresponding oxaaluminacyclopentenes.
Nickel-catalyzed [2+2+2] Cycloaddition of Two Enones and an Alkyne
S. Ogoshi, A. Nishimura, and M. Ohashi
Org. Lett. 2010, 12, 3450-3452.
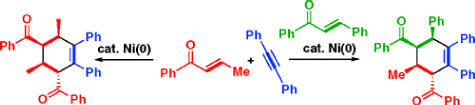
A nickel-catalyzed fully intermolecular [2+2+2] cycloaddition of two enones and an alkyne has been developed. In this reaction, one diastereomer was obtained as a sole product.
[3 + 3] Cyclodimerization of Methylenecyclopropanes: Stoichiometric and Catalytic Reactions of Nickel(0) with Electron-Deficient Alkylidenecyclopropanes.
M. Ohashi, T. Taniguchi and S. Ogoshi
Organometallics 2010, 29, 2386.
Nickel-catalyzed Tishchenko Reaction via Hetero-nickelacycles by Oxidative Cyclization of Aldehydes with Nickel(0) Complex
S. Ogoshi, Y. Hoshimoto and M. Ohashi
Chem. Commun. 2010, 46, 3354.
Nickel-catalyzed Reactions between Enone and Two Ethylenes
S. Ogoshi, A. Nishimura, T. Haba and M. Ohashi
Chem. Lett. 2009, 38, 1166-1167.
Nickel-Catalyzed Direct Conjugate Addition of Simple Alkenes to Enones
S. Ogoshi, T. Haba and M. Ohashi
J.
Am. Chem. Soc. 2009, 131, 10350-10351.
Ni(0)-Catalyzed Formation of Azaaluminacyclopentenes via Azanickelacyclopentenes: A Unique Nickel/Aluminum Double Transmetalation Reaction
M. Ohashi, O. Kishizaki, H. Ikeda and S. Ogoshi
J. Am. Chem. Soc. 2009, 131, 9160-9161.
Synthesis and Reactivity of Six-Membered Oxa-Nickelacycles: Ring-Opening Reaction of Cyclopropyl Ketones
T. Tamaki, M. Nagata, M. Ohashi and S. Ogoshi
Chem. Eur. J. 2009, 15, 10083-10091. (Cover Picture)